Formica exsecta
| Formica exsecta | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Formicini |
| Genus: | Formica |
| Subgenus: | Coptoformica |
| Species: | F. exsecta |
| Binomial name | |
| Formica exsecta Nylander, 1846 | |
| Synonyms | |
| |
This is an active aggressive species building mounds of leaf litter in open woodland, moorland and rough pasture. On disturbance the ants swarm out and bite vigorously. Nests may contain a thousand or more workers. F. exsecta is mainly aphidicolous tending aphids on Juniperus, Picea and other trees but is also predaceous. Colonies extend by nest splitting but single queens also start colonies by securing acceptance in nests of Formica lemani or Formica fusca. Alates occur in July (Collingwood 1979).
| At a Glance | • Temporary parasite • Supercolonies • Polygynous |
Photo Gallery
Identification
Bicoloured with gaster dark brown, rest of body reddish with varying amount of dark colour on head and promesonotum. Head strongly excised posteriorly; maxillary palps 6 segmented, long as half head length. Scale strongly emarginate. Eyes with very distinct erect hairs which are normally abundant. Body pilosity variable - erect hairs on all gaster tergites, on clypeus and on dorsum of head, sometimes also on occipital margins. Clypeus not impressed. Length: 4.5-7.5 mm (Collingwood 1979).
Seifert (2000) - Interspecific setae and pubescence differences between Coptoformica species are normally correlated with differences in body measures and indices at least in the queens. Investigations of the enormous setal and pubescence variability of the exsecta samples seen during this study could not convincingly show such correlations and could not satisfactorily separate entities of possible taxonomic significance. The same local population may show a wide range of pubescence and pilosity variance and there is no clear indication that certain setae morphs could be associated with a certain distribution, habitat selection, or biology. As a consequence and in agreement with the view of Agosti (1989), Formica exsecta is considered here as polymorphic, relatively eurytopic species with a large range, similar to the situation in Formica truncorum, Formica pratensis, and Scandinavian Formica lugubris. This conception of F. exsecta contrasts the situation in the other Coptoformica species that are constant, monomorphic entities with a defined zoogeography and more specific habitat selection. Males: Eyes with numerous long hairs; EyeHL 35-50 μm. Clypeus with numerous setae; ClySet 2-5. Mesosoma with numerous standing setae. Craniad profile of forecoxae with standing setae.
Seifert (2019) provides details for separating this species from the morphologically similar Formica fennica.
Keys including this Species
- Key to Formica subgenus Coptoformica workers of Europe
- Key to Formica subgenus Coptoformica queens of Europe
Distribution
Seifert (2000) - South to Central Spain, to the N Appennine and to the Balkans at 40°N. Found in high Anatolia and Caucasus; apparently absent from the driest Pontic and Caspian steppe zones. West to SW England and the Scottish Highlands. Northern range troughout Fennoscandia up to North Cape. Distribution in the east in European Russia, across Siberia, Mongolia, NE China (Beishan National Park, 37°N, 102°E) and east to the lower Amur river. The northern distribution in the continental parts of Eurasia is limited by the -8 °C isotherm of soil in a depth of one meter (achieved at 67°N in W Siberia at the Ob river and at 62°N in E Siberia at the Lena river) and the southern distribution coincides with the southern border of foreststeppe (Dlussky 1967). Vertical distribution: in Switzerland and Austria 300-2250 m, bimodal, with very low frequencies from 800-1200 m; Bulgarian mountains 1100-2200 m.
The Reinig Line faunal divide separates East Siberian, Inner Mongolian, Chinese and Tibetan species from those of Central Siberia, West Siberia and the Turanian region (DE LATTIN, 1967). In ants, the Reinig Line is crossed only by a cold resistant species including Camponotus herculeanus, Formica exsecta, Formica gagatoides, Formica lugubris, Formica manchu, Formica picea, Formica pisarskii, Formica uralensis, Lasius flavus, Leptothorax acervorum and Tetramorium sibiricum (DLUSSKY, 1967; FRANCOEUR, 1983; SEIFERT, 2000, 2021a, 2021b).
Latitudinal Distribution Pattern
Latitudinal Range: 70.377854° to 36.70419°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Palaearctic Region: Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bulgaria, China, Czechia, Denmark, Estonia, Finland (type locality), France, Germany, Greece, Hungary, Iberian Peninsula, Italy, Kazakhstan, Kyrgyzstan, Latvia, Liechtenstein, Lithuania, Luxembourg, Mongolia, Netherlands, Norway, Poland, North Macedonia, Republic of Moldova, Romania, Russian Federation, Slovakia, Slovenia, Spain, Sweden, Switzerland, Türkiye, Ukraine, United Kingdom of Great Britain and Northern Ireland.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
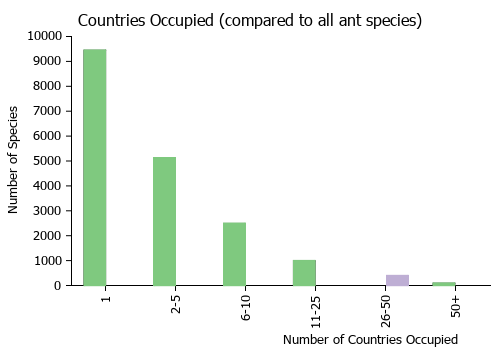
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Habitat
Seifert (2000) - F. exsecta is more a generalist with a wider habitat selection than usual within Coptoformica species. Very different open or slightly shaded habitats, which must have medium-term stability at least, are inhabited. A high cover percentage of grasses within the field layer is typical but not essential. As for all species of the group, sites with rapid plant succession, with high nitrogen input, or sites within the inundation plains of rivers are not colonised. Formica exsecta and all other members of the group cannot increase nest temperatures by metabolic heat production independent of environmental temperatures as known for populous nests of some wood ant species. The dependence of Coptoformica upon direct insolation is thus increased and nests cannot be constructed in fully shaded woodland habitats. Habitat types used by exsecta are subalpine and boreomontane pastures, clearings and margins of woodland, sunny forests, semidry to xerothermous grasslands, heathland, and dryer spots of bogs and fens.
Biology
Goryunov (2015) - Narrow-headed ants mostly inhabit rapidly changing ecotone biotopes. In addition, F. exsecta is obligatorily dominant in multispecies ant assemblages and has to maintain this position from the very foundation of the colony. Thus, this model species has to possess adaptations that would allow it to promptly respond to changes in the environment. Some of these adaptations are realized in its nest-building activity. Simple and movable auxiliary nests facilitate rapid colonization of new areas or help to change the population density in the existing colonies. By relocating auxiliary nests across the foraging area, F. exsecta ants can quickly install supporting points for foragers at the sites rich in food. After the sites are no longer important, ants can take the building material back to the primary nest and do not have to collect the material anew when it is needed. The absence of the inner cone and the propensity for relocation of material allow mature F. exsecta anthills to move together with the forest edge. In addition, building material exchange among formicaries can contribute to integration of different families within the colony, which, in turn, helps to consolidate large complexes with high nesting density.
Status as threatened species
Red List Germany: three (threatened). In Central Europe it is probably the least endangered Coptoformica species though its populations have significantly declined since 1950. The decline is caused by afforestation of clearings and meadows, vanishing of coppice wood management, decline of sheep pasturing, intensifying of cattle pasturing, intensive use of mineral fertilisers and liquid manure, and high athmospheric nitrogen immission. Dewes (1993) recorded F. exsecta colonies in the Nature Park Saar-Hunsrück preferentially in sites of historic coppice wood management where oak bark for tanneries was harvested.
Colony foundation
Flight or ground dispersal of single queens is followed by socially parasitic colony foundation in nests of the subgenus Serviformica. Formica cunicularia (de la Mora et al., 2021; Seifert, 2018), Formica fusca (Dlussky, Collingwood, Pisarski, Agosti, Seifert) and Formica lemani (Collingwood, Agosti, Hellrigl, Seifert) are reported as host species. According to Pisarski (1982), socially parasitic colony foundation is only possible in queenless host nests. The same author stated that monogynous nests of exsecta never accept alien queens, with exception of very minute nests. To become polygynous, they must have lost the queen and can then adopt several queens simultaneously. Once shifted to polygyny, huge polycalic colony systems may develop by nest splitting.
Development and microclimatic requirements
Alates develop from eggs laid in early spring. Oviposition starts in Central European lowland habitats in late March but is considerably delayed with growing altitude and latitude. In Finnish monogynous colonies, less than 5% of the spring eggs develop to workers (Chapuisat et al. 1997), but this ratio may reach 50% in Swiss polygynous societies (Schneider pers. comm.). The main source of worker production is late spring or summer eggs. The optimum temperature for “brood development” is 28-30 °C (Grinfeld 1939, cited in Dlussky 1967). The supercooling point of winter-adapted Siberian workers is -20 °C; the long-term minimum at which 50% of workers survive is -8 °C (Berman, Shigulskaja & Leirich 1987; Leirich 1989). The conditions where soil temperatures in a depth of one metre do not decline below -8 °C are given in E Siberia up to 62°N, which coincides with the northern distributional border of exsecta.
Demography of nests and colonies
The ratio of monogynous nests vs polygynous/polycalic colonies differs locally and geographically. Patchiness and general availability of suitable habitats and a payoff between the costs of single queen colony foundation and the costs of reproductive competition in polygynous nests are probable factors influencing this ratio. In S Finland monogyny/monodomy clearly predominates. Big polycalic colonies are known from the Alps, Central Europe, and European Russia. The mean longevity of queens in monogynous nests in S Finland was over 20 years (Pamilo 1991). According to Sundström et al. (1996), 39% of the queens in 57 monogynous S Finnish nests were multiply-mated (polyandrous). The sex ratio of produced alates is strongly dependent upon the number of queens in a colony and the number of mates per queen; in highly polycalic colonies in the Swiss Alps it was about 15:1 (Schneider pers comm.), in Finnish polyandrous/monogynous colonies it was 3.76:1, but in Finnish monoandrous/monogynous nests it was 1 : 2.2. Workers of the latter colonies increased their inclusive fitness by selectively killing male larvae before pupation and (most probably) feeding them to the now rapidly growing female larvae. Ecological and demographic factors (recource limitation!) are believed to interfere with genetic factors (optimisation of kinship value), i.e. males should survive in higher numbers in monoandrous/monogynous nests if there is plenty of recources (Chapuisat et al. 1997). Monogynous nests mainly produce the large male morph (macraner) but polycalic colonies mainly micraners (Pamilo & Rosengren 1984). The micraners can develop from worker-laid eggs, mature later, and show narrower activity peaks. Both micraners and macraners are normally haploid (Agosti 1989). No complete population census has been performed so far in exsecta. The “Aussendienst” population of four monogynous nests of 368 ± 100 cm2 basal area was censused by Pisarski (1982) as 2750 ± 1000 workers, i.e. 7.5 workers/cm2 basal nest area. That means a total population of 18.8 workers/cm2 basal nest area if assuming 40% Aussendienst workers. The total population of exactly censused polygynous bruni nests was 39.3 workers/cm2 basal nest area (Schneider pers. comm.). Both values seem realistic in view of the higher worker density and smaller worker size in polygynous nests. A very large polygynous exsecta nest of 150 cm diameter should then contain > 300 000 workers. Polycalic exsecta colonies may be huge and can dominate a site as known for Formica polyctena. Dewes (1993) described a supercolony comprising 408 nests (the smallest not counted!) spreading over an area of 2 ha. If assuming only 25 000 workers for an average polygynous exsecta nest, the whole population of this colony should be > 10,000,000.
Swarming
Mature alates are found in the nest 28.2 July ± 20.2 d (10 June-4 Sept, n = 33). In contrast to R. Rosengren, M. A. Schneider did not observe micraners to fly higher and farther than macraners. In Swiss polycalic colonies about 30% of females fly, the others are inseminated at the nest mounds. Swarming is restricted to the the first half of the day (between 5.30 and 12.20 h) and starts as soon as the first direct sunlight hits the mound surface. Completely cloudy sky or strong air movement prevents the flight and beginning sunshine in the second half of the day can not release it (Schneider pers. comm., and my own observations).
Food sources
F. exsecta can use a wide range of food sources. Trophobiosis with epigaeic and subterranean Aphidina (and more rarely Cicadina and Coccina) is observed and obviously covers a major portion of the energy needs. Lachnidae are the main trophobionts in coniferous and deciduous forests. Zoophagy may be important and is then comparable to that of Formica polyctena with the difference of a smaller average size of prey items (Wesselinov & Horstmann 1968); all kinds of dead or living Arthropoda and Lumbricidae are consumed. Very populous supercolonies effectively displace different species of predatory arthropods from their territory.
Intraspecific behaviour
Monogynous colonies are highly aggressive against conspecific aliens. Polygynous/monodomous colonies show reduced aggressivity but only polycalic colonies do not establish territorial boundaries against other conspecific polycalic colonies (Pisarski 1982).
Nesting Habits
Goryunov (2015) - Red wood ants and closely related species build intricate composite aboveground nests with a subterranean part (Dlussky, 1967). The subterranean part is organized similarly to nests located completely underground. The aboveground part is represented by the mound which consists of two elements. Within the mound, there is an inner cone built of relatively large pieces of branches. The inner cone is the backbone of the structure, and this is also where most chambers are situated. It is overlain with a coating cover built of relatively fine plant fragments such as needles, leaves, thin twigs, etc. The mound is elevated on an earthen bank which is built of soil evacuated during the construction of the subterranean part of the nest.
Formica exsecta also build nests with aboveground mounds that include plant fragments (Dlussky, 1967). Their nests look similar to formicaries of red wood ants but differ considerably in the inner structure and the preferred building material, while the peculiar lifestyle of F. exsecta is mirrored in the way in which they use the nest.
The primary structural feature of F. exsecta formicaries, as opposed to red wood ant nests, is that in the former the inner cone is absent. This puts considerable limitations on the size, and first of all height, of F. exsecta anthills. In some cases, F. exsecta ants are able to compensate for the absence of the inner cone by building their nests over various objects, such as stones, logs, young trees, etc.
The second distinguishing feature of F. exsecta nest mounds is different building material. While red wood ants mostly build mounds of needles and branch fragments, F. exsecta ants prefer pieces of herbs and grasses, leaves of shrubs and trees, and lichen thalli (Dmitrienko and Petrenko, 1976). During this work, ants can cut leaf fragments of different size (Goryunov, 2012). The finest pieces of plants are used by F. exsecta for encrusting soil particles which are then fitted into the mound.
A significant amount of soil in the mound itself is yet another essential feature of F. exsecta nests. The underlying bank of the anthill is usually not pronounced, since much soil is used for erecting the mound. Conspicuous banks were only observed by me in Kostroma Province. Several large nests were positioned on “pedestals” that did not look like natural hillocks and were presumably built by ants to avoid overshadowing of the growing colony by tall grass stand.
Soil particles are fitted into the peripheral layers of the mound more densely and in greater amounts than in the center. This results in a hard crust layer 1–1.5 cm thick which protects the colony from rainfall and gives shape to the nest, i.e., serves as armature. The crust layer was absent in all the nests from the upper reaches of the Kolyma River due to a very thin soil layer in that region. For the most part of the growing season, the crust layer is covered with freshly collected plant material (not mixed with soil) which forms a temporary layer. The latter layer is completely incorporated into the former during preparation for winter.
There is an intermediate layer between the mound and the subterranean part of the nest of F. exsecta, which is absent in red wood ant nests. This layer almost exclusively consists of large chambers whose walls are made of soil mixed with numerous fine plant fragments. The intermediate layer is somewhat elevated above the soil surface, its thickness being 6–8 cm.
There is one more group of large chambers in F. exsecta nests. It is situated above, under the vault of the mound. In small anthills, this group is substituted by a single chamber which is very wide and was previously described as the main chamber (Dlussky, 1967) or as the brood chamber (Dmitrienko and Petrenko, 1976).
The mound itself, compared with the crust, consists of a less densely packed and less soil-rich material. Deep within the mound, there are horizontally positioned chambers which are smaller than the uppermost ones.
In summer, the upper chambers and those around the vertical axis of the nest are filled with brood which is sorted by age. Pupae occupy the uppermost chambers with successively younger stages deeper down. Eggs are situated just above the intermediate layer. In addition, the closer chambers are to the intermediate layer, the less they are filled with brood, i.e., the brood rearing zone forms an inverted cone relative to the mound.
Auxiliary Nests
In addition to living nests, F. exsecta ants use auxiliary nests for various purposes. The mound in such nests contains only plant material, because ants do not dig soil in this case. In other words, these nests lack the subterranean part, the intermediate layer, and, as a rule, the crust. Due to the peculiarities of structure and utilization, these nests rarely exceed the diameter of 0.25 m at the bottom. Ants often surround sedge and grass stems with picked and excised plant fragments so that the tussock acts as a backbone to the auxiliary nest.
Auxiliary nests may be temporary and exist only for one season or relatively permanent. Temporary auxiliary nests are more common in large developed formicary complexes of F. exsecta. In such complexes, tens of auxiliary nests emerge every year, and the fraction of auxiliary nests reaches up to 50% of all anthills. Ants often erect series of 2–3 auxiliary nests that form a line running along the sunniest edge of the primary nest (Goryunov, 2007).
The main functions of temporary auxiliary nests are structuring of the foraging area, maintenance of food transport chains (Pisarski, 1972), and provision of shelters for remote foragers. Ants use series of auxiliary nests to choose an optimal site for such a nest (after a month, only one nest from the series remains) or for a new primary nest. When colonizing a new foraging area, F. exsecta ants start with building a network of temporary auxiliary nests and only afterwards they erect primary formicaries. The position of primary nests in the new area may not coincide with the position of initial auxiliary nests. In spring, buffering auxiliary nests may emerge between primary formicaries as relationships between the latter become restored. In case of especially tight relationships, a series of auxiliary nests or a single attenuate gallery-like nest may exist between the primary nests during the whole season.
Besides that, temporary auxiliary nests are used by F. exsecta ants in the process of construction of primary anthills. In this case, the auxiliary nest is built in the vicinity of the main nest or even on its edge. Then the auxiliary nest gradually becomes connected with the primary one by means of a gallery until the mound of the primary nest completely overlays the auxiliary nest. This process may be repeated more than once, which eventually results in a teardrop-shaped anthill.
The functions of permanent auxiliary nests largely coincide with main functions of temporary ones. Permanent auxiliary nests emerge in F. exsecta formicary complexes when environmental conditions and the colony’s structure in the entire complex or in its part remain stable over a long period of time. Most commonly permanent auxiliary nests are built near constant sources of aphid colonies (e.g., trees, shrubs, and perennial herbs). When a permanent auxiliary nest exists long enough it may get covered with crust, but neither the intermediate layer, nor the subterranean part ever appear in such nests.
Relocation of Building Material
It is shown for red wood ant nests that building materials are being constantly relocated within the anthill (Kloft, 1959). F. exsecta ants also move nest material from one place in the anthill to another. However, while in red wood ants the bank and the inner cone determine the shape of the anthill, in F. exsecta the crust layer does so. Therefore, manipulations with nest material in F. exsecta are all the more important.
The most favorable habitats for F. exsecta are rapidly changing transitional ones, such as forest edges, glades, and clearings. Under such conditions, it is important to be able to quickly change the configuration of the nest. By changing the configuration of the crust layer, F. exsecta ants can rather promptly respond to changes in the environment. In the course of the season, the anthill bottom may change its shape from round to oval, or vice versa. An additional top may appear or disappear on the anthill.
Besides that, whole F. exsecta nests can be moved continuously or step-like. At some time, an anthill may become constantly overshadowed by growing vegetation. In such cases, the primary F. exsecta nest may travel a distance of about 1.5 m in several weeks with only the intermediate layer and subterranean part left at the former site. Gradual relocations of primary anthills may last for years with several centimeters traveled each year. After a few years, an anthill may occupy a position where previously there was another one.
Relocation of building material within the nest is easily observable once the nest has been damaged in summer. At this time, the crust is covered with fresh material. When some part of the mound is damaged, ants peel all of the fresh material off and transfer it to the damaged region.
Relocation of building material in F. exsecta ants is not limited to within-nest movements. The mound material, except for the intermediate layer, is an object of exchange between other anthills. This is best observed in manipulations with auxiliary nests.
Auxiliary nests of F. exsecta can be easily erected and dismantled since they consist of plant fragments only. If the auxiliary nest is no longer needed, the ants take its mound apart. The building material can be used for the primary anthill or other auxiliary nests.
The same takes place when ants, for whatever reason, have to leave their primary nest. All of the mound material from the abandoned nest is transferred to one or more neighboring anthills or brought to a new site. Only the intermediate layer and the subterranean part remain in the place of the abandoned nest. Ants may visit the remaining parts of the nest and maintain their functionality. Subsequently a new mound may be erected in the site of the old nest, either over the whole intermediate layer or on a part of it. In any case, abandoned mounds exist for only a short time in developed F. exsecta colonies. Only in colonies situated in the upper reaches of the Kolyma did I observe evidently old abandoned mounds of F. exsecta ants. However, in this region, mounds are almost exclusively built of larch needles that quickly deteriorate as nest material. In addition, anthill mounds at the upper Kolyma contain excised fragments of lichen thalli. There were no lichens in the inspected abandoned nests, which suggests that, for F. exsecta ants, excised material is more valuable than picked-up one.
Besides relocation of the mound and redistribution of material among auxiliary nests, colonies of F. exsecta exhibit background exchange of materials among primary nests. The speed of this exchange may be considerable. Between two neighboring anthills situated 10–20 cm apart (from one bottom edge to the other), transfer of nest material from the most remote edge of one nest to the most remote edge of the other takes about 12 h.
Relocation of building material within the nest and between large (over 60 cm in diameter at the bottom) neighboring anthills may lead to fusion of these nests. There are two scenarios of fusion. When two nests gradually grow or move, at some time their mound bottoms may come into contact. Once this has happened, extensive building and widening of the mounds begin at the joint. Subsequently the two nests may unite under a common mound or retain both mounds. In the future, other neighboring anthills may join the new common nest as well. According to the second scenario, ants build a gallery that connects the mounds of fusing anthills. Underneath the gallery, the intermediate layer and the subterranean part are formed. Subsequently the gallery may be extended and two anthills may fuse as in the first scenario or, alternatively, anthills will remain connected by means of the gallery for quite a long time. It should be noted that fusion by means of a gallery is reversible. After some time, ants may dismantle the gallery and only the intermediate layer and the subterranean part will remain, which will perish after a year without maintenance. I never observed disconnection of the nests that had fused according to the first scenario or any other kind of gradual division of nests.
Seasonal Aspects of Nest-Building
The pattern and intensity of nest-building activity change during the season. Leaf fragments are mostly excised by ants from mid-May to early June; later this behavior is so weakly pronounced that it is almost impossible to find the characteristic damage to grasses and herbs around the anthill.
Approximately three weeks after the ants start to excise plant fragments, the rapid growth of primary anthills and construction of new ones begin. New nests are usually founded before the first third of July. However, if a formicary complex has invaded a new area, construction of new nests may proceed till the end of July, but only in these new territories.
In the middle of and late in the season, the total nest bottom area in F. exsecta colonies practically does not increase. During this period, large formicary complexes lose most of their temporary auxiliary nests. It is common after the swarming of alates (as a rule, early in August) that the total nest bottom area may even decrease. However, nests and their groups may still be changing in size almost till the end of the season.
The middle and the end of the season is a time when ant abundance is redistributed across the whole formicary complex. The movement of ants is accompanied by relocation of nest material and may occur between the neighboring nest groups or in a relay manner from one edge of the complex to the other. In some seasons, redistribution of abundance may affect over one-third of all the inhabitants of the complex (Goryunov, 2009).
Seifert (2000) - Nest mounds are often more structured than in related species. Materials and type of nest construction depend upon insolation, ground water level, soil type, and composition of vegetation. The following type is frequent on mineralic soils: a calotte-like outer hull of the dome, which can often be lifted without breaking (Agosti 1989), is constructed with more dense, strongly adhesive materials (usually finely cut grass pieces). Rather voluminous upper brood chambers (during warm weather the sites for pupae) may be situated between this hull and inner mound material that consists of more lofty plant pieces. The lower mound core is a mixture of humified plant material and mineralic soil. Galleries may reach 150 cm down into the soil. In polygyous nests and during summer, numerous clearly separated chambers of 2 cm width are found below the level of soil surface down to 80 cm. These chambers usually contain a queen, eggs, small larvae, and few workers. In habitats deficient of grasses, other materials as coniferous needles, rabbit pellets, or small pebbles may be used. Nest diameters may reach 200 or even 300 cm (Agosti 1989; Dlussky 1967). In semi-shaded boreal forests, the author observed nest mounds of 150 cm height and 180 cm diameter, the base of which had been abandoned by the ants and covered by mosses (Polytrichum).
Genetics
Gyllenstrand et al. (2002) identified fourteen polymorphic microsatellite loci for the study of genetic population structure and mating structure across the varying types of social organization exhibited by this species (Seppa et al. 2004).
Flight Period
| X | X | X | |||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
 Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a xenobiont for the ant Formicoxenus nitidulus (a xenobiont) (Holldobler & Wilson 1990; Busch 2001; Martin et al. 2007).
- This species is a host for the beetle Monotoma angusticollis (Coleopotera: Monotomidae) (a myrmecophile) in Europe (Wagner et al., 2020).
- This species is a host for the phorid fly Aenigmatias lubbocki (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
Fungi
- This species is a host for the fungus Aegeritella superficialis (Espadaler & Santamaria, 2012).
- This species is a host for the fungus Pandora myrmecophaga (a pathogen) in Romania (Csata et al., 2013).
- This species is a host for the fungus Pandora formicae (a pathogen) (Małagocka et al., 2017).
Life History Traits
- Queen number: polygynous (Pamilo & Rosengren, 1984; Frumhoff & Ward, 1992)
- Queen type: winged (Pamilo & Rosengren, 1984; Frumhoff & Ward, 1992) (queenless worker reproduction)
- Queen mating frequency: multiple (Pamilo & Rosengren, 1984; Frumhoff & Ward, 1992)
- Colony type: supercolony
- Compound colony type: temporary parasite
- Colony founding: social parasite
- Nest site: thatch mound
Castes
Worker
Queen
Images from AntWeb
    
| |
| Queen (alate/dealate). Specimen code casent0173162. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Male
Images from AntWeb
   
| |
| Male (alate). Specimen code casent0010687. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by UCDC, Davis, CA, USA. |
     
| |
| Male (alate). Specimen code casent0173163. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- exsecta. Formica exsecta Nylander, 1846a: 909, pl. 18, fig. 20 (w.q.m.) FINLAND. Combination in F. (Coptoformica): Müller, 1923: 146. Senior synonym of dalcqi, etrusca: Bernard, 1967: 323; of exsectopressilabris: Bernard, 1967: 323; Dlussky & Pisarski, 1971: 194; of kontuniemii, sudetica, wheeleri Stitz: Dlussky, 1967a: 100; Dlussky & Pisarski, 1971: 194; of rubens: Dlussky, 1964: 1027; of nemoralis: Seifert, 2000a: 526. See also: Donisthorpe, 1915d: 273; Parapura, 1972: 763; Kutter, 1977c: 283; Agosti & Hauschteck-Jungen, 1988: 280; Kupyanskaya, 1990: 200; Atanassov & Dlussky, 1992: 282; Seifert, 2000a: 525; Radchenko, 2007: 36.
- exsectopressilabris. Formica exsecta var. exsectopressilabris Forel, 1874: 52 (w.q.m.) SWITZERLAND. Subspecies of pressilabris: Dalla Torre, 1893: 205. Raised to species: Bondroit, 1912: 352; Bondroit, 1918: 63. Subspecies of exsecta: Karavaiev, 1927c: 284; Karavaiev, 1936: 255; Boven, 1947: 189. Junior synonym of exsecta: Bernard, 1967: 323; Dlussky & Pisarski, 1971: 194.
- rubens. Formica exsecta var. rubens Forel, 1874: 51 (w.) SWITZERLAND. Raised to species: Bondroit, 1918: 62. Subspecies of exsecta: Ruzsky, 1925b: 43; Emery, 1925b: 257. Junior synonym of exsecta: Dlussky, 1964: 1027.
- etrusca. Formica exsecta var. etrusca Emery, 1909b: 191, fig. 5 (w.) ITALY. Raised to species: Bondroit, 1918: 64. Subspecies of exsecta: Emery, 1925b: 257; Karavaiev, 1926e: 196. Junior synonym of exsecta: Bernard, 1967: 323.
- dalcqi. Formica dalcqi Bondroit, 1918: 63 (w.) FRANCE. Subspecies of exsecta: Emery, 1925b: 257. Junior synonym of exsecta: Bernard, 1967: 323.
- sudetica. Formica exsecta var. sudetica Scholz, 1924: 48 (w.) POLAND. Junior synonym of exsecta: Dlussky & Pisarski, 1971: 194.
- kontuniemii. Formica kontuniemii Betrem, 1954: 230 (w.) FINLAND. Junior synonym of exsecta: Dlussky, 1967a: 100; Dlussky & Pisarski, 1971: 194.
- nemoralis. Formica nemoralis Dlussky, 1964: 1037 (w.m.) UKRAINE. Junior synonym of forsslundi: Dlussky, 1967a: 105; of exsecta: Seifert, 2000a: 526.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Borowiec and Salata (2022) - Large, HL: 1.241-1.556 (mean 1.414); HW: 1.048-1.381 (mean 1.188); SL: 1.270-1.540 (mean 1.328); EL: 0.354-0.571 (mean 0.511); ML: 1.64-2.07; MW: 0.74- 0.94. Color. Head bicolours, clypeus, genae, sides behind eyes and ventral side yellowish to yellowish red, rest of surface brown to black, pale anterior part of head gradually turn into the dark posterior part of head without sharp border between pale and dark parts; mesosoma usually uniformly yellowish to yellowish red, occasionally pronotum at top with obscure spot of diffused borders, petiolar scale usually yellowish to yellowish red, gaster brown to black with transparent white posterior margin of tergites, anterior slope of first tergite often yellowish brown, antennae yellowish red to red, sometimes apical antennomeres gradually infuscate, legs usually completely yellowish to yellowish brown. Head. Broad, 1.1-1.3 times longer than wide, in front of eyes softly converging anterad, behind eyes softly rounded, occipital margin deeply emarginate. Clypeus without or with obtuse median keel, on the whole surface distinctly microsculptured, slightly trapezoidal, its anterior margin convex, sides convergent posterad, posterior margin truncate, whole clypeal surface with very short and sparse appressed pubescence, clypeal margin with 3 long setae centrally and 4-5 short setae laterally, usually also with a row of 4 setae in anterior part, sometimes with only two setae anteriorly, occasionally also with a pair of erected setae close to base, the longest anterior seta with length 0.190. Head distinctly microreticulate, appears dull and opaque, with very short and very sparse appressed pubescence not covering head surface, erected setae absent or with a pair of short erected setae in interocular area and in ocellar area. Scape moderately long, approximately 1.1 times as long as width of head, thin, distinctly reaching beyond the occipital margin, distinctly, regularly widened from base to apex, its surface microreticulate, with short and dense appressed pubescence, erected setae absent. Funicular segments elongate, thin, first segment 1.7 times as long as second segment, the second segment 1.7 times as long as wide, only slightly shorter than third segment, the rest of funicular segments clearly longer than broad. Eyes big, elongate oval, approximately 0.27 length of head. Mesosoma. Elongate in dorsal view distinctly constricted in the middle, 2.1-2.4 times as long as wide, dorsally and laterally distinctly microreticulated, surface indistinctly dull and opaque. In lateral view promesonotum convex, mesonotal groove deep, propodeum strongly, obtusely convex. Whole mesosomal surface covered with short and sparse appressed pubescence not covering the mesosomal surface, erected setae absent. Waist and gaster. Petiolar scale moderately broad, moderately thick in lateral view, apex straight but with deep median emargination, without setae. Gaster shorter than mesosoma, all tergites distinctly microreticulate, appears dull and opaque, covered with short and sparse appressed pubescence not covering surface of tergites. Tergites 1-2 or only tergite 2 with a row of setae, sometimes reduced to 2-3 setae, tergite 3 with a row of setae close to posterior margin and few erected setae on dorsal surface. Legs. Ventral surface of fore and mid femora with lacking erected setae.
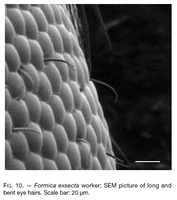
Seifert (2000) - Maximum size larger than in other species (CL 1419 ± 82, 1200-1641; CW 1362 ± 83, 1131-1574). Head shape of average Coptoformica type (CL/CW 1.042 ± 0.023, 0.979-1.099); however, long-headed and short-headed specimens may occur within the same nest. Rather long scape (SL/CL 1.008 ± 0.022, 0.931-1.063). Clypeus at least in anterior third, but normally also in median and posterior portions with standing setae (ClySet 3.54 ± 1.08, 2-5). Lateral semierect setae in the ocellar triangle usually present (OceSet 92%). Eye hairs at least in a fraction of the nest population strongly developed, often hook-shaped (EyeHL 27.0 ± 6.5, 0-45; Fig. 10). Pubescence in the occellar triangle frequently dilute, but enormous intraspecific and intranidal variation occurs (sqrtPDF 5.69 ± 0.90, 3.78-9.30). Region of occipital corners with semierect to subdecumbent pubescence (however, in specimens of the etrusca population almost appressed). Craniad profile of forecoxae with semierect setae (nCOXA 8.86 ± 3.89, 0.5-23). Dorsal mesosoma and propodeum occasionally with few standing setae, lateral metapleuron and ventrolateral propodeum more frequently setaceous (nMET 1.86 ± 2.11, 0-9). Outer edge of the hind tibial flexor side conspicuously hairy (nHTFL 10.97 ± 2.82, 5.0-23.0), with two size classes of setae, and subdecumbent pubescence (Fig. 2). Semierect setae on gaster tergites as a rule beginning on the 1st tergite (TERG 1.19 ± 0.46, 1-3); nest sample means of TERG always < 2.4. Pubescence density on first gaster tergite with extreme intranidal and intraspecific variation (sqrtPDG 6.82 ± 1.19, 3.93-9.88).
Queen
Seifert (2000) - Size definitely larger than in other species (CL 1636 ± 44, 1514-1741; CW 1721 ± 42, 1629-1809; ML 2878 ± 116, 2613-3115). Head broad (CL/CW 0.950 ± 0.022, 0.900-1.008), scape long (SL/CL 0.956 ± 0.023, 0.893-1.004). Clypeus at least in anterior third, but normally also in median and posterior portions with standing setae. Lateral semierect setae in the ocellar triangle normally present. Eye hairs normally long and numerous, often hook-shaped (EyeHL 45.6 ± 7.6, 31-69); samples with less numerous eye hairs may occur. Pubescence in the occellar triangle relatively dense (sqrtPDF 4.28 ± 0.49, 3.34-5.75), less variable than in workers. Occipital corners of head normally with semierect smaller setae and pubescence, morphs with almost reduced and such with very developed occipital hairs may occur (OccHD 46.9 ± 22.5, 7-107). Brilliance of dorsal head surface variable, but relatively matt and weakly sculptured surfaces dominate (GLANZ 1.71 ± 0.36, 1.0-2.5). Craniad profile of forecoxae with semierect setae (nCOXA 12.95 ± 4.09, 3.5-23.0). Promesonotum normally with standing setae that clearly differ from semierect pubescence, in weakly haired specimens the differentiation between shorter semierect setae and longer semierect pubescence can be lost (MnHL 181.8 ± 40.0, 0-256). Outer edge of the hind tibial flexor side conspicuously hairy (nHTFL 12.81 ± 3.20, 8.0-22.0), with two size classes of setae and subdecumbent pubescence. Semierect setae on gaster tergites always beginning on the first tergite (TERG 1.00 ± 0.00). Pubescence density on first gaster tergite with extreme intraspecific variation (sqrtPDG 6.17 ± 1.16, 3.83-9.25).
Type Material
- Syntype, 3 workers, 1 queen, 1 male, Helsinki, Finland, Field Museum of Natural History.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- n = 26, 2n = 52 (Switzerland) (Agosti & Hauschteck-Jungen, 1987; Hauschteck-Jungen & Jungen, 1976).
References
- Agosti, D.; Hauschteck-Jungen, E. 1988 [1987]. Polymorphism of males in Formica exsecta Nyl. (Hymenoptera: Formicidae). Insectes Soc. 34: 280-290 (page 280, see also)
- Alexandrovna, K.A. 2020. Red wood ants (Formica s. str.) as a method of biological protection in phytocenoses of the Mordovia Republic. In: Vavilov Readings - 2020: a collection of articles of the International Scientific and Practical Conference dedicated to the 100th anniversary of the discovery of the law of homological series and the 133rd anniversary of the birth of Academician NI Vavilov, November 24-25, 2020.
- Antonov, I. A., Bukin, Yu. S. 2016. Molecular phylogenetic analysis of the ant genus Formica L. (Hymenoptera: Formicidae) from Palearctic region. Russian Journal of Genetics, 52(8), 810–820 (doi:10.1134/s1022795416080020).
- Atanassov, N.; Dlussky, G. M. 1992. Fauna of Bulgaria. Hymenoptera, Formicidae. Fauna Bûlg. 22: 1-310 (page 282, see also)
- Baty, J.W., Bulgarella, M., Dobelmann, J., Felden, A., Lester, P.J. 2020. Viruses and their effects in ants (Hymenoptera: Formicidae). Myrmecological News 30: 213-228 (doi:10.25849/MYRMECOL.NEWS_030:213).
- Beresford, J. 2021. The role of hybrids in the process of speciation; a study of naturally occurring Formica wood ant hybrids. Academic Dissertation, University of Helsinki.
- Berkelhamer, R.C. 1980. Reproductive strategies in ants: A comparison of single-queened versus multiple-queened species in the subfamily Dolichoderinae (Hymenoptera: Formicidae). Ph.D. thesis, University of California, Berkeley.
- Bernadou, A., Fourcassié, V., Espadaler, X. 2013. A preliminary checklist of the ants (Hymenoptera, Formicidae) of Andorra. ZooKeys 277, 13–23 (doi:10.3897/zookeys.277.4684).
- Bernard, F. 1967a [1968]. Faune de l'Europe et du Bassin Méditerranéen. 3. Les fourmis (Hymenoptera Formicidae) d'Europe occidentale et septentrionale. Paris: Masson, 411 pp. (page 323, senior synonym of dalcqi, etrusca, senior synonym of exsectopressilabris)
- Boer, P. 2008. Observations of summit disease in Formica rufa Linnaeus, 1761 (Hymenoptera: Formicidae). Myrmecological News 11. 63-66.
- Borowiec, L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Borowiec, L., Salata, S. 2022. A monographic review of ants of Greece (Hymenoptera: Formicidae). Vol. 1. Introduction and review of all subfamilies except the subfamily Myrmicinae. Part 1: text. Natural History Monographs of the Upper Silesian Museum 1: 1-297.
- Borowiec, M.L., Cover, S.P., Rabeling, C. 2021. The evolution of social parasitism in Formica ants revealed by a global phylogeny. Proceedings of the National Academy of Sciences 118, e2026029118 (doi:10.1073/pnas.2026029118).
- Boudinot, B. E., Moosdorf, O. T. D., Beutel, R. G., Richter, A. 2021. Anatomy and evolution of the head of Dorylus helvolus (Formicidae: Dorylinae): Patterns of sex‐ and caste‐limited traits in the sausagefly and the driver ant. Journal of Morphology 282(11), 1616–1658 (doi:10.1002/jmor.21410).
- Bracko, G., Wagner, H.C., Schulz, A., Gioahin, E., Maticic, J., Trantnik, A. 2014. New investigation and a revised checklist of the ants (Hymenoptera: Formicidae) of the Republic of Macedonia. North-Western Journal of Zoology 10: 10-24.
- Brassard, F., Francoeur, A., Lessard, J.P. 2020. Temperature drives caste‐specific morphological clines in ants. Journal of Animal Ecology 89, 2517–2530. (doi:10.1111/1365-2656.13330).
- Brelsford, A., Purcell, J., Avril, A., Tran Van, P., Zhang, J., Brütsch, T., Sundström, L., Helanterä, H., Chapuisat, M. 2020. An ancient and eroded social supergene is widespread across Formica ants. Current Biology 30, 304–311 (doi:10.1016/j.cub.2019.11.032).
- Bulter, I. 2020. Hybridization in ants. Ph.D. thesis, Rockefeller University.
- Caliari Oliveira, R., van Zweden, J., Wenseleers, T. 2021. Similarities in recognition cues lead to the infiltration of non-nestmates in an ant species. Journal of Chemical Ecology (doi:10.1007/s10886-021-01325-3).
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Cantone, S., Von Zuben, C.J. 2019. The hindwings of ants: A phylogenetic analysis. Psyche: A Journal of Entomology 2019, 1–11 (doi:10.1155/2019/7929717).
- Collingwood, C. A. 1979. The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomol. Scand. 8:1-174.
- Csata, E., Czekes, Z., Eros, K., Nemet, E., Hughes, M., Csosz, S., Marko, B. 2013. Comprehensive survey of Romanian myrmecoparasitic fungi: new species, biology and distribution. North-western Journal of Zoology 9: 23-29.
- Csősz, S., Báthori, F., Gallé, L., Lőrinczi, G., Maák, I., Tartally, A., Kovács, É., Somogyi, A.Á., Markó, B. 2021. The myrmecofauna (Hymenoptera: Formicidae) of Hungary: Survey of ant species with an annotated synonymic inventory. Insects 16;12(1):78 (doi:10.3390/insects12010078).
- Csosz, S., Marko, B., Galle, L. 2011. The myrmecofauna (Hymenoptera: Formicidae) of Hungary: an updated checklist. North-Western Journal of Zoology 7: 55-62.
- Czechowski, W., Radchenko, A. 2006. Formica lusatica SEIFERT, 1997 (Hymenoptera: Formicidae), an ant species new to Finland, with notes on its biology and the description of males. Myrmecologische Nachrichten 8: 257-262.
- Czechowski, W., Radchenko, A., Czechowska, W. 2002. The ants (Hymenoptera, Formicidae) of Poland. MIZ PAS Warsaw.
- de la Mora, A., Sankovitz, M., Purcell, J. 2020. Ants (Hymenoptera: Formicidae) as host and intruder: recent advances and future directions in the study of exploitative strategies. Myrmecological News 30: 53-71 (doi:10.25849/MYRMECOL.NEWS_030:053).
- Dekoninck, W., Ignace, D., Vankerkhoven, F., Wegnez, P. 2012. Verspreidingsatlas van de mieren van België. Bulletin de la Société royale belge d’Entomologie 148: 95-186.
- Dekoninck, W., Maebe, K., Breyne, P., Hendrickx, F. 2014. Polygyny and strong genetic structuring within an isolated population of the wood ant Formica rufa. Journal of Hymenoptera Research 41, 95–111 (doi:10.3897/jhr.41.8191).
- Dekoninck, W., Wauters, N., Delsinne, T. 2019. Capitulo 35. Hormigas invasoras en Colombia. Hormigas de Colombia.
- Dlussky, G. M. 1964. The ants of the subgenus Coptoformica of the genus Formica (Hymenoptera, Formicidae) of the USSR. Zool. Zh. 4 43: 1026-1040 (page 1027, senior synonym of rubens)
- Dlussky, G. M. 1967a. Ants of the genus Formica (Hymenoptera, Formicidae, g. Formica). Moskva: Nauka Publishing House, 236 pp. (page 100, senior synonym of kontunienmii, sudetica and wheeleri)
- Dlussky, G. M.; Pisarski, B. 1971. Rewizja polskich gatunków mrówek (Hymenoptera: Formicidae) z rodzaju Formica L. Fragmenta Faunistica 16: 145-224 (page 194, senior synonym of exsectopressilabris, senior synonym of kontunienmii, sudetica and wheeleri)
- Donisthorpe, H. 1915f. British ants, their life-history and classification. Plymouth: Brendon & Son Ltd., xv + 379 pp. (page 273, see also)
- Dubovikoff, D.A., Yusupov, Z.M. 2017. Family Formicidae - Ants. In Belokobylskij S. A. and A. S. Lelej: Annotated catalogue of the Hymenoptera of Russia. Proceedingss of the Zoological Institute of the Russian Academy of Sciences 6: 197-210.
- Espadaler, X., Santamaria, S. 2012. Ecto- and Endoparasitic Fungi on Ants from the Holarctic Region. Psyche Article ID 168478, 10 pages (doi:10.1155/2012/168478).
- Fournier, D., de Biseau, J.-C., De Laet, S., Lenoir, A., Passera, L., Aron, S. 2016. Social structure and genetic distance mediate nestmate recognition and aggressiveness in the facultative polygynous ant Pheidole pallidula. PLOS ONE 11, e0156440. (doi:10.1371/journal.pone.0156440).
- García, F., Cuesta-Segura, A.D., Espadaler, X. 2024. Myrmica babiensis sp. nov. (Hymenoptera: Formicidae), a new social parasite from the NW Iberian Peninsula. Annales Zoologici 74(1), 113-127 (doi:10.3161/00034541anz2024.74.1.006).
- Godfrey, R.K., Oberski, J.T., Allmark, T., Givens, C., Hernandez-Rivera, J., Gronenberg, W. 2021. Olfactory system morphology suggests colony size drives trait evolution in Odorous Ants (Formicidae: Dolichoderinae). Frontiers in Ecology and Evolution 9, 733023 (doi:10.3389/fevo.2021.733023).
- Goropashnaya, A.V., Fedorov, V.B., Seifert, B., Pamilo, P. 2012. Phylogenetic relationships of Palaearctic Formica species (Hymenoptera, Formicidae) based on mitochondrial Cytochrome b sequences. PLoS ONE 7, e41697 (doi:10.1371/journal.pone.0041697).
- Goryunov, D. N. 2015. Nest building in Formica exsecta ants (Hymenoptera, Formicidae). Zoologichesky Zhurnal. 94:1132-1137. doi:10.1134/S0013873815080035
- Gyllenstrand, N., P. J. Gertsch, and P. Pamilo. 2002. Polymorphic microsatellite DNA markers in the ant Formica exsecta. Molecular Ecology Notes. 2(1):67-69. doi:10.1046/j.1471-8286.2002.00152.x
- Helantera, H., Sundstrom, L. 2007. Worker reproduction in Formica ants. The American Naturalist 170: E15-E25.
- Imai, H.T., Kihara, A., Kondoh, M., Kubota, M., Kuribayashi, S., Ogata, K., Onoyama, K., Taylor, R.W., Terayama, M., Yoshimura, M., Ugawa, Y. 2003. Ants of Japan. 224 pp, Gakken, Japan.
- Jacobs, S. 2020. Population genetic and behavioral aspects of male mating monopolies in Cardiocondyla venustula (Ph.D. thesis).
- Kiran, K., Karaman, C. 2020. Additions to the ant fauna of Turkey (Hymenoptera, Formicidae). Zoosystema 42(18), 285-329 (doi:10.5252/zoosystema2020v42a18).
- Kummerli R, Keller L (2007) Contrasting population genetic structure for workers and queens in the putatively unicolonial ant Formica exsecta: population genetic structure in ants. Mol Ecol 16:4493–4503. doi:10.1111/j.1365-294X.2007.03514.x
- Kupyanskaya, A. N. 1990a. Ants of the Far Eastern USSR. Vladivostok: Akademiya Nauk SSSR, 258 pp. (page 200, see also)
- Kutter, H. 1977c. Hymenoptera, Formicidae. Insecta Helv. Fauna 6: 1-298 (page 283, see also)
- Maák, I., Czekes, Z., Erős, K., Pálfi, Z., Markó, B. 2020. Living on the edge: Changes in the foraging strategy of a territorial ant species occurring with a rival supercolony – a case study. Journal of Insect Behavior 33, 59–68 (doi:10.1007/S10905-020-09745-X).
- Małagocka, J., Jensen, A.B., Eilenberg, J. 2017. Pandora formicae, a specialist ant pathogenic fungus: New insights into biology and taxonomy. Journal of Invertebrate Pathology 143, 108–114 (doi:10.1016/j.jip.2016.12.007).
- Meurville, M.-P., LeBoeuf, A.C. 2021. Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae). Myrmecological News 31: 1-30 (doi:10.25849/MYRMECOL.NEWS_031:001).
- Müller, G. 1923b. Le formiche della Venezia Guilia e della Dalmazia. Boll. Soc. Adriat. Sci. Nat. Trieste 28: 11-180 (page 146, combination in F. (Coptoformica))
- Nouhaud, P., Beresford, J., Kulmuni, J. 2022. Assembly of a hybrid Formica aquilonia × F. polyctena ant genome from a haploid male. Journal of Heredity, 113(3), 353–359 (doi:10.1093/jhered/esac019).
- Nouhaud, P., Martin, S.H., Portinha, B., Sousa, V.C., Kulmuni, J. 2022. Rapid and predictable genome evolution across three hybrid ant populations. PLOS Biology, 2012, e3001914 (doi:10.1371/journal.pbio.3001914).
- Nylander, W. 1846a. Adnotationes in monographiam formicarum borealium Europae. Acta Societatis Scientiarum Fennicae. 2:875-944. (page 909, pl. 18, fig. 20 worker, queen, male described)
- Oswalt, D.A. 2007. Nesting and foraging characteristics of the black carpenter ant Camponotus pennsylvanicus DeGeer (Hymenoptera: Formicidae). Ph.D. thesis, Clemson University.
- Pamilo, P., Sundström, L., Fortelius, W., Rosengren, R. 1994. Diploid males and colony-level selection in Formica ants. Ethology Ecology, Evolution 6, 221–235 (doi:10.1080/08927014.1994.9522996).
- Parapura, E. 1972. An ergatandromorph Formica exsecta Nyl. (Hymenoptera, Formicidae) from Poland. Bull. Acad. Pol. Sci. Sér. Biol. 20: 763-767 (page 763, see also)
- Pulliainen, U., Helantera, H., Sundstrom, L., Schultner, E. 2019. The possible role of ant larvae in the defence against social parasites. Proceedings of the Royal Society B: Biological Sciences 286: 20182867 (doi:10.1098/rspb.2018.2867).
- Punttila, P., Kilpeläinen, J. 2009. Distribution of mound-building ant species (Formica spp., Hymenoptera) in Finland: preliminary results of a national survey. Annales Zoologici Fennici 46: 1–15.
- Putyatina, T.S., Gilev, A.V., Grinkov, V.G., Markov, A.V. 2022. Variation in the colour pattern of the narrow-headed ant Formica exsecta (Hymenoptera: Formicidae) in European Russia. European Journal of Entomology 119, 58-68 (doi:10.14411/eje.2022.006).
- Schifani, E. (2022). The new checklist of the Italian fauna: Formicidae. Biogeographia – The Journal of Integrative Biogeography 37, ucl006 (doi:10.21426/b637155803).
- Schultner, E., Pulliainen, U. 2020. Brood recognition and discrimination in ants. Insectes Sociaux 67, 11–34 (doi:10.1007/s00040-019-00747-3).
- Seifert, B 2000a. A taxonomic revision of the ant subgenus Coptoformica Mueller, 1923 (Hymenoptera: Formicidae). Zoosystema 22:517-568. (page 526, Senior synonym of nemoralis)
- Seifert, B. 2019. The Rubens morph of Formica exsecta Nylander, 1846 and its separation from Formica fennica Seifert, 2000 (Hymenoptera, Formicidae). Deutsche Entomologische Zeitschrift 66: 55-61 (doi:10.3897/dez.66.34868).
- Seifert, B. 2021. Surviving the winter: Tetramorium sibiricum n. sp., a new Central Siberian ant species (Hymenoptera: Formicidae). Osmia 9, 15–24 (doi:10.47446/osmia9.3).
- Seifert, B., Schultz, R. 2021. A taxonomic revision of the Palaearctic ant subgenus Coptoformica Müller, 1923 (Hymenoptera, Formicidae). Beiträge zur Entomologie 71 (2): 177–220 (doi:10.21248/contrib.entomol.71.2.177-220).
- Seppä, P., N. Gyllenstrand, J. Corander, and P. Pamilo. 2004. Coexistence of the social types: genetic population structure in the ant Formica exsecta. Evolution. 58(11):2462-2471. doi:10.1111/j.0014-3820.2004.tb00875.x
- Siedlecki, I., Gorczak, M., Okrasińska, A., Wrzosek, M. 2021. Chance or necessity—The fungi co−occurring with Formica polyctena ants. Insects 12, 204 (doi:10.3390/insects12030204).
- Silva, P.S., Koch, E.B. de A., Arnhold, A., Delabie, J.H.C. 2022. Review of distribution modeling in ant (Hymenoptera: Formicidae) biogeographic studies. Sociobiology 69(4), e7775 (doi:10.13102/sociobiology.v69i4.7775).
- Ślipiński, P., Trigos-Peral, G., Maák, I., Wojciechowska, I., Witek, M. 2021. The influence of age and development temperature on the temperature-related foraging risk of Formica cinerea ants. Behavioral Ecology and Sociobiology 75, 107 (doi:10.1007/s00265-021-03029-w).
- Sundstrom, L. 1993. Genetic population structure and sociogenetic organisation in Formica truncorum (Hymenoptera; Formicidae). Behavioral Ecology and Sociobiology 33:345-354
- Wagner, G.K., Staniec, B., Zagaja, M., Pietrykowska-Tudruj, E. 2020. First insight into detailed morphology of monotomids, with comments on chaetotaxy and life history based on myrmecophilous Monotoma angusticollis. Bulletin of Insectology 73 (1): 11-27.
- Walin, L., Sundstrom, L., Seppa, P., Rosengren, R. 1998. Worker reproduction in ants — a genetic analysis. Heredity 81, 604–612.
References based on Global Ant Biodiversity Informatics
- Agosti, D. and C.A. Collingwood. 1987. A provisional list of the Balkan ants (Hym. Formicidae) and a key to the worker caste. I. Synonymic list. Mitteilungen der Schweizerischen Entomologischen Gesellschaft, 60: 51-62
- Agosti, D. and E.Hauschteck-Jungen. 1988. Polymorphism of males in Formica exsecta Nyl. (Hymenoptera: Formicidae). Insectes Sociaux, 34: 280-290.
- AntArea. Accessed on February 5th 2014 at http://antarea.fr/fourmi/
- Antarea (Personal Communication - Rumsais Blatrix- 27 April 2018)
- ArtDatabanken Bugs (via GBIG)
- Asociacion Iberica de Mirmecologia. 2011. List of species collected during the Taxomara Lisboa 2011. Iberomyrmex 3: 30-31.
- Azuma M. 1977. On the myrmecological-fauna of Mt. Rokko, Hyogo, with description of a new species (Formicidae, Hymenoptera). Hyogo Biology 7:112-118.
- Banert P, and B. Pisarski. 1972. Mrówki (Formicidae) Sudetów. Fragmenta Faunistica (Warsaw) 18: 345-359.
- Baroni Urbani C., and C. A. Collingwood. 1976. A Numerical Analysis of the Distribution of British Formicidae (Hymenoptera, Aculeata). Verhandlungen der Naturforschenden Gesellschaft in Basel 85: 51-91.
- Baroni Urbani C., and C. A. Collingwood. 1977. The zoogeography of ants (Hymenoptera, Formicidae) in Northern Europe. Acta Zoologica Fennica 152: 1-34.
- Bernadou A., V. Fourcassié, and X. Espadaler. 2013. A preliminary checklist of the ants (Hymenoptera, Formicidae) of Andorra. Zookeys 277: 13-23.
- Bernard F. 1967. Faune de l'Europe et du Bassin Méditerranéen. 3. Les fourmis (Hymenoptera Formicidae) d'Europe occidentale et septentrionale. Paris: Masson, 411 pp.
- Bezdecka P. 1996. The ants of Slovakia (Hymenoptera: Formicidae). Entomofauna carpathica 8: 108-114.
- Blinov V. V. 1984. New for the fauna of Byelorussia ant species. Vestsi Akademii Navuk BSSR. Seryia Biialahichnykh Navuk 1984(5): 113-115.
- Boer P. 2019. Species list of the Netherlands. Accessed on January 22 2019 at http://www.nlmieren.nl/websitepages/specieslist.html
- Boer P., W. Dekoninck, A. J. Van Loon, and F. Vankerkhoven. 2003. Lijst van mieren (Hymenoptera: Formicidae) van Belgie en Nederland, hun Nederlandse namen en hun voorkomen. Entomologische Berichten (Amsterdam) 63: 54-58.
- Boer P., W. Dekoninck, A. J. van Loon, and F. Vankerkhoven. 2003. Lijst van mieren (Hymenoptera: Formicidae) van Belgie en Nederland, hun Nederlandse namen en hun voorkomen. Entomologische Berichten 63(3): 54-57.
- Boer P., W. Dekoninck, A. J. van Loon, and F. Vankerkhoven. 2003. List of ants (Hymenoptera: Formicidae) of Belgium and The Netherlands, their status and Dutch vernacular names. Entomologische Berichten 63 (3): 54-58.
- Borowiec L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Borowiec L., and S. Salata. 2012. Ants of Greece - Checklist, comments and new faunistic data (Hymenoptera: Formicidae). Genus 23(4): 461-563.
- Boven J. van 1949. Notes sur la faune des Hautes-Fagnes en Belgique. Bulletin et Annales de la Société Entomologique de Belgique 85: 135-143.
- Bracko G. 2007. Checklist of the ants of Slovenia (Hymenoptera: Formicidae). Natura Sloveniae 9: 15-24
- Brown, W.D. and L. Keller. 2006. Resource Supplements Cause a Change in Colony Sex-Ratio Specialization in the MoundBuilding Ant, Formica exsecta. Behavioral Ecology and Sociobiology 60(5):612-618
- Brown, W.D., L. Keller and L. Sundström. 2002. Sex Allocation in Mound-Building Ants: The Roles of Resources and Queen Replenishment. Ecology 83(7):1945-1952
- Carniel A. 1998. Ricerche sulla mirmecofauna delle Prealpi Orobiche (Lombardia) (Insecta, Hymenoptera, Formicidae). Atti. Mus. Civ. Stor. Nat. Morbegno 9: 29-39.
- Casevitz-Weulersse J., and C. Galkowski. 2009. Liste actualisee des Fourmis de France (Hymenoptera, Formicidae). Bull. Soc. Entomol. Fr. 114: 475-510.
- Casevitz-Weulersse J., and M. Prost. 1991. Fourmis de la Côte-d'Or présentes dans les collections du Muséum d'Histoire Naturelle de Dijon. Bulletin Scientifique de Bourgogne 44: 53-72.
- Cherix D., and S. Higashi. 1979. Distribution verticale des fourmis dans le Jura vaudois et recensement prelimaire des bourdons (Hymenoptera, Formicidae et Apidae). Bull. Soc. Vaud. Sc. Nat. 356(74): 315-324.
- Collingwood C. A. 1961. Ants in the Scottish Highlands. Scotish Naturalist 70: 12-21.
- Collingwood C. A. 1971. A synopsis of the Formicidae of north Europe. Entomologist 104: 150-176
- Collingwood C.A. 1959. Ants in the Scottish Highlands. The Scottish Naturalist. 70: 12-21
- Collingwood C.A. 1959. Scandinavian Ants. Entomol. Rec. 71: 78-83
- Collingwood C.A. 1961. Ants in Finland. Entomol. Rec. 73: 190-195
- Collingwood C.A. 1961. New Vice-County Records for British Ants. Entomologist. 73: 90-93
- Collingwood, C. A. 1958b. A key to the species of ants (Hymenoptera, Formicidae) found in Britain. Trans. Soc. Br. Entomol. 13: 69-96
- Collingwood, C. A. 1964. The Identification of British Ants (Hym. Formicidae). Transactions of the Society for British Entomology. 16:93-121.
- Collingwood, C. A. 1974. A revised list of Norwegian ants (Hymenoptera: Formicidae). Norsk Entomologisk Tidsskrift 21: 31-35.
- Collingwood, C. A.. "The Formicidae (Hymenoptera) of Fennoscandia and Denmark." Fauna Entomologica Scandinavica 8 (1979): 1-174.
- Csősz S., B. Markó, and L. Gallé. 2011. The myrmecofauna (Hymenoptera: Formicidae) of Hungary: an updated checklist. North-Western Journal of Zoology 7: 55-62.
- Czechowski W., A. Radchenko, W. Czechowska and K. Vepsäläinen. 2012. The ants of Poland with reference to the myrmecofauna of Europe. Fauna Poloniae 4. Warsaw: Natura Optima Dux Foundation, 1-496 pp
- Dahms H., L. Lenoir, R. Lindborg, V. Wolters, and J. Dauber. 2008. Restoration of seminatural grasslands: what is the impact on ants? Restoration Ecology 18(3): 330-337.
- Dauber, J., J. Bengtsson and L. Lenoir. 2006. Evaluating Effects of Habitat Loss and Land-Use Continuity on Ant Species Richness in Seminatural Grassland Remnants. Conservation Biology 20(4):1150-1160
- Della Santa E. 1994. Guide pour l'identification des principales espèces de fourmis de Suisse. Miscellanea Faunistica Helvetiae 3: 1-124.
- Donisthorpe H. 1914. Myrmecophilous notes for 1913. Entomologist's Record and Journal of Variation 26: 37-45.
- Donisthorpe H. 1929. The Formicidae (Hymenoptera) taken by Major R. W. G. Hingston, M.C., I.M.S. (ret.), on the Mount Everest Expedition, 1924. Annals and Magazine of Natural History (10)4: 444-449.
- Dubovikoff D. A., and Z. M. Yusupov. 2018. Family Formicidae - Ants. In Belokobylskij S. A. and A. S. Lelej: Annotated catalogue of the Hymenoptera of Russia. Proceedingss of the Zoological Institute of the Russian Academy of Sciences 6: 197-210.
- Else G., B. Bolton, and G. Broad. 2016. Checklist of British and Irish Hymenoptera - aculeates (Apoidea, Chrysidoidea and Vespoidea). Biodiversity Data Journal 4: e8050. doi: 10.3897/BDJ.4.e8050
- Emery C. 1916. Fauna entomologica italiana. I. Hymenoptera.-Formicidae. Bullettino della Società Entomologica Italiana 47: 79-275.
- Emery, C.. "Catalogo delle formiche esistenti nelle collezioni del Museo Civico di Genova. Parte seconda. Formiche dell'Europa e delle regioni limitrofe in Africa e in Asia." Annali del Museo Civico di Storia Naturale 12 (1878): 43-59.
- Entomological Society of Latvia. 2003. http://leb.daba.lv/Formicidae.htm (Accessed on December 1st 2013).
- Espadaler, X.. "Contribución al conocimiento de los formícidos (Hymenoptera, Formicidae) del Pirineo catalán." Tesis Universida (1979): 285 pp.
- Forel A. 1892. Die Ameisenfauna Bulgariens. (Nebst biologischen Beobachtungen.). 305-318.
- Formidabel Database
- Gallé L., B. Markó, K. Kiss, E. Kovács, H. Dürgő, K. Kőváry, and S. Csősz. 2005. Ant fauna of Tisza river basin (Hymenoptera: Formicidae). In: Gallé, L. (szerk.): Vegetation and Fauna of Tisza River Basin I. Tiscia Monograph Series 7; Szeged, pp. 149-197.
- Garcia Garcia F., and A. D. Cuesta-Esgura. 2017. First catalogue of the ants of Burgos province, Spain (Hymenoptera: Formicidae). Boletín de la Sociedad Entomológica Aragonesa 60: 245–258.
- Gibb H., and T. Johansson. 2011. Field tests of interspecific competition in ant assemblages: revisiting the dominant red wood ants. Journal of Animal Ecology 80: 548-557.
- Gilev A. V., I. V. Kuzmin, V. A. Stolbov, and S. D. Sheikin. 2012. Materials on the fauna and ecology of ants (formicidae) Southern part of the Tyumen region. Tyumen State University Herald 6: 86-91.
- Grandi G. 1935. Contributi alla conoscenza degli Imenotteri Aculeati. XV. Boll. R. Ist. Entomol. Univ. Studi Bologna 8: 27-121.
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Haag-Liautard, C., J. S. Pedersen, O. Ovaskainen and L. Keller. 2008. Breeding system and reproductive skew in a highly polygynous ant population. Insectes Sociaux 55(4):347-354.
- Hayashida K. 1971. Vertical distribution of ants in the southern part of the Hidaka mountains. [In Japanese.]. Memoirs of the National Science Museum (Tokyo) 4:29-38.
- Hayashida K. 1972. Ecological survey on ants in Nakagawa Experiment Forest of Hokkaido University. Res. Bull. Exper. Forests, Coll. Agr., Hokkaido Univ. 29: 25-36.
- Heatwole H. 1989. Changes in ant assemblages across an artic treeline. Revue d'Entomologie du Quebec 34(1-2): 10-22.
- Holgersen H. 1942. Ants of northern Norway (Hym., Form.). Tromso Mus. Årsh. 63(2): 1-34.
- Holgersen H. 1943. Ant studies in Rogaland (south-western Norway). Avhandlingar utgitt av det Norske Videnskaps-Akademi i Oslo. I. Matematisk-Naturvidenskapelig Klasse 1943(7): 1-75.
- Holgersen H. 1944. The ants of Norway (Hymenoptera, Formicidae). Nytt Magasin for Naturvidenskapene 84: 165-203.
- Holgersen H. 1944. The ants of Norway (Hymenoptera, Formicidae). Nytt Magasin for Naturvidenskapene 84: 165-291.
- Holgersen H. 1944. The ants of Norway (Hymenoptera, Formicidae). Nytt Magasin for Naturvidenskapene 84: 165-450.
- Holzer, B., M. Chapuidat and L. Keller. 2008. Foreign ant queens are accepted but produce fewer offspring. Behavioral Ecology and Sociobiology 157:717-723
- Hågvar S. 2005. Altitudinal zonation of ants (Formicidae) in a steep fjord landscape in Sogndal, Western Norway. Norw. J. Entomol. 52: 3-12.
- IZIKO South Africa Museum Collection
- Jeffery H. G. 1931. The Formicidae (or ants) of the Isle of Wight. Proceedings of the Isle of Wight Natural History and Archaeological Society 2: 125-128.
- Karaman M. G. 2009. An introduction to the ant fauna of Macedonia (Balkan Peninsula), a check list (Hymenoptera, Formicidae). Natura Montenegrina 8(3): 151-162.
- Karaman M. G. 2011. A catalogue of the ants (Hymenoptera, Formicidae) of Montenegro. Podgorica: Catalogues 3, Volume 2, Montenegrin Academy of Sciences and Arts, 140 pp.
- Katzerke, A., P. Neumann, C.W.W. Pirk, P. Bliss and R. A. Moritz. 2006. Seasonal Nestmate Recognition in the Ant Formica exsecta. Behavioral Ecology and Sociobiology 61(1) :143-150
- Kiran K., and C. Karaman. 2012. First annotated checklist of the ant fauna of Turkey (Hymenoptera: Formicidae). Zootaxa 3548: 1-38.
- Kofler A. 1995. Nachtrag zur Ameisenfauna Osttirols (Tirol, Österreich) (Hymenoptera: Formicidae). Myrmecologische Nachrichten 1: 14-25.
- Kvamme T. 1982. Atlas of the Formicidae of Norway (Hymenoptera: Aculeata). Insecta Norvegiae 2: 1-56.
- Lameere A. 1892. Note sur les fourmis de la Belgique. Annales dr la Société Entomologique de Belgique 36: 61-69.
- Lapeva-Gjonova, L., V. Antonova, A. G. Radchenko, and M. Atanasova. "Catalogue of the ants (Hymenoptera, Formicidae) of Bulgaria." ZooKeys 62 (2010): 1-124.
- Lauterer P. 1968. Notes on the occurrence of four rare species of ants in Moravia. Ac. Rer. Natur. Mus. Nat. Slov., Bratislava 54(1): 95-98.
- Le Moli F., and A. Zaccone. 1995. Ricerche sulla mirmecofauna del Cansiglio (Prealpi Carniche). Soc. Ven. Sc. Nat. 20: 33-52.
- Legakis A. 2001. Ants (Hymenoptera, Formicidae) collected in the eastern Pyrenees and surrounding area, September 1999. Proceedings of the International Colloqium of the European Invertebrate Survey: Cartography and Conservation of Biodiversity Marcevol Priory, Arboussols, france 30.8-4.9.1999. OPIE.
- Legakis Collection Database
- Lelej A. S. 2012. Annotated catalogue of the Insects of Russian Far East. Volume 1. Hymenoptera. Dalnauka: Vladivostok. 635 p.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Liaturd, C., W.D. Brown, K.R. Helms and L. Keller. 2003. Temporal and Spatial Variations of Gyne Production in the Ant Formica exsecta. Oecologia 136(4):558-564
- Maavara V. 1953. Ants of Estonian SSR. ABIKS loodusevaatlejale 10: 1-44.
- Majzlan O., and P. Devan. 2009. Selected insect groups (Hymenoptera, Neuroptera, Mecoptera, Raphidioptera) of the Rokoš Massif (Strážovské vrchy Mts.). Rosalia (Nitra), 20, p. 63–70.
- Malozemova L. A. 1972. Ants of steppe forests, their distribution by habitats, and perspectives of their utilization for protection of forests (north Kazakhstan). [In Russian.]. Zoologicheskii Zhurnal 51: 57-68.
- Markó B., B. Sipos, S. Csősz, K. Kiss, I. Boros, and L. Gallé. 2006. A comprehensive list of the ants of Romania (Hymenoptera: Formicidae). Myrmecologische Nachrichten 9: 65-76.
- Martin, S.J., H. Helanterä, K. Kiss, Y. R. Lee and F. P. Drijfhout. 2009. Polygyny reduces rather than increases nestmate discrimination cue diversity in Formica exsecta ants. Insectes Sociaux 56(4):375-383.
- Mizutani A. 1979. A myrmecofaunal survey at Hiyama Experiment Forest, Hokkaido University. Research Bulletin of the College Experiment Forests, Hokkaido University 36:509-516.
- Nadig A. 1918. Alcune note sulla fauna dell'alta Valsesia. Formicidae. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 56: 331-341.
- Neumeyer R., and B. Seifert. 2005. Commented check list of free living ant (Hymenoptera: Formicidae) species of Switzerland. Bulletin de la Societe Entomologique Suisse 78: 1-17.
- Nylander, W.. "Synopsis des Formicides de France et d'Algérie." Annales des Sciences Naturelles, Zoologie (4)5 (1856): 51-109.
- Odegaard F. 2013. New and little known ants (Hymenoptera, Formicidae) in Norway. Norwegian Journal of Entomology 60, 172175.
- Parapura E. 1972. An ergatandromorph Formica exsecta Nyl. from Poland. Bulletin de l'Academie Polonaise des Sciences (2) 20: 763-767.
- Paraschivescu D. 1978. Elemente balcanice in mirmecofauna R. S. Romania. Nymphaea 6: 463- 474.
- Paukkunen J., and M. V. Kozlov. 2015. Stinging wasps, ants and bees (Hy menoptera: Aculeata) of the Murmansk region, Northwest Russia. — Entomol. Fennica. 26: 53–73.
- Petal J. M. 1963. Faune des fourmis de la reserve de tourbiere en projet a Rakowskie Bagno pres de Frampol (voivodie de Lublin). Annales Universitatis Mariae Curie-Sk?odowska 58(7): 143-174.
- Petal J. M. 1967. Contribution a la connaissance des fourmis (Formicidae, Hymenoptera) de la region de Lublin. VII. Associations des fourmis des milieux de tourbieres, de forets et de dunes aux environs de Libiszow (dist. De Parczew). Annales Universitatis Mariae Curie-Sklodowska Lublin-Polonia 22(9): 117-130.
- Petrov I. Z., and C. A. Collingwood. 1992. Survey of the myrmecofauna (Formicidae, Hymenoptera) of Yugoslavia. Archives of Biological Sciences (Belgrade) 44: 79-91.
- Punttila P., and Y. Haila. 1996. Colonisation of a burned forest by ants in the southern Finnish Boreal forest. Silva Fennica 30(4): 421-435.
- Pusvaskyte O. 1979. Myrmecofauna of the Lituanian SSR. Acta Entomologica Lituanica 4: 99-105.
- Ran H., and S. Y. Zhou. 2012. Checklist of chinese ants: formicomorph subfamilies (Hymenoptera: Formicidae) II. Journal of Guangxi Normal University: Natural Science Edition 30(4): 81-91.
- Reznikova Z. I. 2003. Distribution patterns of ants in different natural zones and landscapes in Kazakhstan and West Siberia along a meridian trend. Euroasian Entomological Journal 2(4): 235-342.
- Rigato F., and R. Sciaky. 1989. Contributo alla conoscenza della mirmecofauna della Val Gesso (alpi Marittime) (Hymenoptera, Formicidae). Boll. Mus. Reg. Sci. Nat. Torino 7(2): 427-442.
- Rigato F., and R. Sciaky. 1991. The myrmecofauna of the Gesso Valley (Maritime Alps) (Hymenoptera Formicidae). Ethology Ecology and Evolution Special Issue 1: 87-89.
- Ruzsky M. 1916. On zoological research in Yeniseisk province, work of summer of 1915. Izv. Imp. Tomsk. Univ. 65 (3rd p part: 1-21.
- Röszler P. 1950. Die Ameisenwelt des Nagy Pietrosz, 2305 m (Ungarn) und Umgebung. Zool. Anz. 145: 210-225.
- Saure C. 2005. Rote Liste und Gesamtartenliste der Bienen und Wespen (Hymenoptera part.) von Berlin mit Angaben zu den Ameisen. In: DER LANDESBEAUFTRAGTE FÜR NATURSCHUTZ UND LANDSCHAFTSPFLEGE / SENATSVERWALTUNG FÜR STADTENTWICKLUNG (Hrsg.): Rote Listen der gefährdeten Pflanzen und Tiere von Berlin. CD-ROM.
- Schlick-Steiner B. C., and F. M. Steiner. 1999. Faunistisch-ökologische Untersuchungen an den freilebenden Ameisen (Hymenoptera: Formicidae) Wiens. Myrmecologische Nachrichten 3: 9-53.
- Schultz R., and B. Seifert. 2007. The distribution of the subgenus Coptoformica Müller, 1923 (Hymenoptera: Formicidae) in the Palaearctic Region. Myrmecological News 10: 11-18.
- Seifert B. 1994. Die freilebenden Ameisenarten Deutschlands (Hymenoptera: Formicidae) und Angaben zu deren Taxonomie und Verbreitung. Abhandlungen und Berichte des Naturkundemuseums Görlitz 67(3): 1-44.
- Seifert B. 1998. Rote Liste der Ameisen. - in: M. Binot, R. Bless, P. Boye, H. Gruttke und P. Pretscher: Rote Liste gefährdeter Tiere Deutschlands. Bonn-Bad Godesberg 1998: 130-133.
- Seifert B. 2000. A taxonomic revision of the ant subgenus Coptoformica Mueller, 1923 (Hymenoptera, Formicidae). Zoosystema 22: 517-568.
- Seifert B. 2007. Die Ameisen Mittel- und Nordeuropas. Tauer: lutra Verlags- und Vertriebsgesellschaft, 368 pp.
- Seifert B. 2019. The Rubens morph of Formica exsecta Nylander, 1846 and its separation from Formica fennica Seifert, 2000 (Hymenoptera, Formicidae). Deutsche Entomologische Zeitschrift 66: 55-61.
- Seppa, P., N. Gyllenstrand, J. Corander and P. Pamilo. 2004. Coexistence of the Social Types: Genetic Population Structure in the Ant Formica exsecta. Evolution 58(11):2462-2471
- Siberian Zoological Museum. Website available at http://szmn.sbras.ru/old/Hymenop/Formicid.htm. Accessed on January 27th 2014.
- Sonnenburg H. 2005. Die Ameisenfauna (Hymenoptera: Formicidae) Niedersachsens und Bremens. Braunschweiger Naturkundliche Schriften 7: 377-441.
- Sonobe, R.; Dlussky, G. M. 1977. On two ant species of the genus Formica (Hymenoptera, Formicidae) from Japan. Kontyû 45:24
- Sonobe, R.; Dlussky, G. M. 1977. On two ant species of the genus Formica (Hymenoptera, Formicidae) from Japan. Kontyû 45:25
- Steiner F. M., S. Schödl, and B. C. Schlick-Steiner. 2002. Liste der Ameisen Österreichs (Hymenoptera: Formicidae), Stand Oktober 2002. Beiträge zur Entomofaunistik 3: 17-25.
- Stumper R. 1953. Etudes myrmecologiques. XI. Fourmis luxembourgeoises. Bulletin Soc. Nat. luxemb. 57: 122-135.
- Sundström, L., L. Keller and M. Chapuisat. 2003. Inbreeding and Sex-Biased Gene Flow in the Ant Formica exsecta. Evolution 57(7):1552-1561
- Suvak M. 2013. First record of Formica fennica Seifert, 2000 (Hymenoptera, Formicidae) in Norway. Norwegian Journal of Entomology 60: 73–80.
- Suñer i Escriche, David. "Contribució al coneixement mirmecologic de Gavarres, Montgrí, Guilleríes i la Serralada Transversal." Tesis Doctoral Universida (1991): 577 pp.
- Tang J., Li S., Huang E., Zhang B. and Chen Y.. 1995. Hymenoptera: Formicidae (1). Economic Insect Fauna of China 47: 1-133.
- Terayama. M. 2004. Geological and ecological distribution of Japanese ants communities. (translated from Japanese) Reports of the Saitama Prefecture Animal Research Association. 48:24
- Vepsalainen K., H. Ikonene, and M. J. Koivula. 2008. The structure of ant assembalges in an urban area of Helsinki, southern Finland. Ann. Zool. Fennici 45: 109-127.
- Vespalainen K., B. Pisarski, R. Kantorek, and K. J. Laine. 1984. Formicidae (Hymenoptera) of Inari Lapland. Kevo Notes 7: 115-116.
- Wegnez P., and M. Fichaux. 2015. Liste actualisee des especes de fourmis repertoriees au Grand-Duche de Luxembourg (Hymenoptera: Formicidae). Bulletin de la Société royale belge d’Entomologie 151: 150-165
- Wheeler W. M. 1913. A revision of the ants of the genus Formica (Linné) Mayr. Bulletin of the Museum of Comparative Zoology 53: 379-565.
- Wu wei, Li Xiao Mei, Guo Hong. 2004. A primary study on the fauna of Formicidae in Urumqi and its vicinities. Arid Zone Research 21(2): 179-182
- Zhuytszyuan D. 2016. The ants (Hymenoptera, Formicidae) Nizhne-Svirsky reserve and their environmental features. Master's thesis Saint Petersburg State University.
- Zryanin V. A., and T. A. Zryanina. 2007. New data on the ant fauna Hymenoptera, Formicidae in the middle Volga River Basin. Uspekhi Sovremennoi Biologii 127(2): 226-240.
- Pages using DynamicPageList3 parser function
- Temporary parasite
- Supercolonies
- Polygynous
- Photo Gallery
- North temperate
- Ant Associate
- Host of Formica cunicularia
- Host of Formica fusca
- Host of Formica lemani
- Nesting Notes
- FlightMonth
- Host of Formicoxenus nitidulus
- Beetle Associate
- Host of Monotoma angusticollis (Coleopotera: Monotomidae)
- Phorid fly Associate
- Host of Aenigmatias lubbocki
- Fungus Associate
- Host of Aegeritella superficialis
- Host of Pandora myrmecophaga
- Host of Pandora formicae
- Karyotype
- Species
- Extant species
- Formicidae
- Formicinae
- Formicini
- Formica
- Coptoformica
- Formica exsecta
- Formicinae species
- Formicini species
- Coptoformica species
- Formica species
- Ssr