Dinoponera
| Dinoponera | |
|---|---|

| |
| Dinoponera australis | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Alliance: | Pachycondyla genus group |
| Genus: | Dinoponera Roger, 1861 |
| Type species | |
| Ponera grandis, now Dinoponera grandis) | |
| Diversity | |
| 8 species (Species Checklist, Species by Country) | |
Dinoponera is a strictly South American genus in the subfamily Ponerinae, tribe Ponerini, commonly called tocandiras or giant Amazonian ants (Zahl 1959, Fourcassié and Oliviera 2002, Haddad et al. 2005). These ants are generally less well known than Paraponera clavata, the bullet ant or hormiga bala (Haddad et al. 2005), yet Dinoponera workers may exceed 3cm in total body length, making them the largest in the world. The genus has been found from montane rainforest on the eastern slope of the Andes in Perú, Ecuador and Colombia to savannah and lowland rainforest in Brazil, Guyana, south through Bolivia, Paraguay and Argentina. (Lenhart, Dash & Mackay, 2013.) The genus is also notable for reproducing via gamergates (with the complete loss of the queen caste).
| At a Glance | • Gamergate |
Photo Gallery
 Dinoponera australis. Photo by Guilherme Ide.
Dinoponera australis. Photo by Guilherme Ide.
Identification
Lenhart, Dash & Mackay (2013) - Size (TBL > 2.5cm) can easily distinguish Dinoponera from other worker ants. Two laterally projecting clypeal teeth and rows of spines on the pygidium and hypopygidium will further distinguish this genus. The gamergates of Dinoponera are not distinct from workers in their external morphology (Haskins and Zahl 1971, Araujo et al. 1990, Paiva and Brandão 1995, Monnin and Peeters 1998). True gynes have not been found in this genus.
Schmidt and Shattuck (2014) - Dinoponera workers are unmistakable due to their enormous size. Other diagnostic characters (in combination) include: subtriangular mandibles, clypeal teeth, complex metapleural gland orifice, toothed tarsal claws, and stout hypopygial spines. Some of these characters are synapomorphic with Pachycondyla, which is sister to Dinoponera and the most similar genus morphologically. Pachycondyla lacks the huge size, subtriangular mandibles, clypeal teeth, and toothed tarsal claws of Dinoponera. Streblognathus bears some resemblance to Dinoponera, given its large size, subtriangular mandibles, clypeal teeth, and forward facing eyes, but Streblognathus has a novel fin-shaped petiole and lacks the complex metapleural gland orifice, toothed tarsal claws, and hypopygial spines of Dinoponera, and is somewhat smaller.
| See images of species within this genus |
Keys including this Genus
Keys to Species in this Genus
Distribution
Dinoponera is a strictly South American genus, found from montane rainforests on the eastern slope of the Andes in Perú, Ecuador and Colombia to savannah and lowland rainforest in Brazil, Guyana, south through Bolivia, Paraguay and Argentina (Lenhart et al., 2013). Lenhart et al. also provide maps showing the distribution of each Dinoponera species.
Distribution and Richness based on AntMaps
Species by Region
Number of species within biogeographic regions, along with the total number of species for each region.
| Afrotropical Region | Australasian Region | Indo-Australian Region | Malagasy Region | Nearctic Region | Neotropical Region | Oriental Region | Palaearctic Region | |
|---|---|---|---|---|---|---|---|---|
| Species | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 |
| Total Species | 2841 | 1736 | 3045 | 932 | 835 | 4379 | 1741 | 2862 |
Biology
Schmidt and Shattuck (2014) - Dinoponera is one of the best studied ponerine genera, due to its interesting social behaviors and large body size, which makes it relatively easy to work with. In particular, the papers by Monnin and colleagues document in unprecedented detail the social and reproductive behaviors of Dinoponera, especially of Dinoponera quadriceps (e.g., Monnin et al., 1998, 2002, 2003; Monnin & Peeters, 1997, 1998, 1999; Monnin & Ratnieks, 1999, 2001; Peeters et al., 1999; Hart & Monnin, 2006; also Hart & Ratnieks, 2005). We will only briefly summarize their results here, with the caveat that much of this information stems from studies of D. quadriceps alone, and its general applicability to the entire genus is in some cases uncertain.
Dinoponera colonies generally contain fewer than 100 workers, though colony size varies among species (Fowler, 1985; Paiva & Brandão, 1995; Fourcassié & Oliveira, 2002; Vasconcellos et al., 2004; Monnin & Peeters, 2008). Dinoponera has completely lost the queen caste and reproduction in each colony is instead performed by a single gamergate (mated egg-laying worker) who is the top (alpha) individual in a ranked dominance hierarchy (Haskins & Zahl, 1971; Monnin et al., 2003). Below her in the hierarchy are three to five subordinate workers who vie with one another for the opportunity to succeed the alpha once she dies (Monnin et al., 2002; Peixoto et al., 2008). These hopeful reproductives perform little work in the colony and represent a drain on the colony’s resources (Monnin & Ratnieks, 1999, 2001; Monnin et al., 2003; Hart & Monnin, 2006). The remaining workers perform most of the labor for the colony and also police the colony, punishing high-ranking workers who attempt to prematurely overthrow the alpha (Monnin & Ratnieks, 2001; Monnin et al., 2002). This policing is quite effective, as early replacement of the alpha is apparently rare (Hart & Monnin, 2006).
A newly annointed alpha worker briefly leaves the colony and mates with a single male (Monnin & Peeters, 1998). Subordinate workers do not mate, but sometimes lay haploid eggs which will develop into males if the alpha does not discover and cannibalize them (Dantas de Araujo et al., 1990; Monnin & Ratnieks, 2001). Alpha workers have a different Cuticular Hydrocarbons profile from other workers (Monnin et al. 1998; Peeters et al., 1999), and this profile is transferred to their eggs, allowing the eggs of subordinates to be identified and eaten by the gamergate (Monnin & Peeters, 1997). The hydrocarbon profile is linked to ovarian activity, and allows workers to assess the rank and reproductive status of colony members (Monnin et al., 1998; Monnin & Peeters, 1999; Peeters et al., 1999; Monnin & Ratnieks, 2001).
As with most ponerines lacking winged queens, colony reproduction in Dinoponera occurs via fission, with workers carrying brood and males to new nest sites and recruiting other workers via tandem running, with apparently no chemical trail (Dinoponera australis: Fowler, 1985; Dinoponera gigantea: Overal, 1980). Nests are built into the soil, can be quite extensive, and house diverse communities of myrmecophiles including inquiline Pheidole species (Zahl, 1957; Hermann et al., 1994; Paiva & Brandão, 1995; Vasconcellos et al., 2004). Workers are not generally aggressive but can deliver a painful sting if provoked (Allard, 1951; Zahl, 1957; Hermann et al., 1994). Dinoponera workers forage diurnally or crepuscularly on the ground, and are generalist predators of insects and opportunistic scavengers of fruits and other food sources (Oldham et al., 1994; Hermann et al., 1994; Paiva & Brandão, 1995; Fourcassié & Oliveira, 2002; Monnin et al., 2003; Araújo & Rodrigues, 2006). Workers always forage individually (Zahl, 1957; Haskins & Zahl, 1971; Fowler, 1985; Fourcassié & Oliveira, 2002; Araújo & Rodrigues, 2006). Orientation and navigation by foraging D. gigantea workers were studied by Fourcassié et al. (1999).
The large size and interesting behaviors of Dinoponera have made them attractive model systems for histological and biochemical research, including studies of the mandibular gland (Oldham & Morgan, 1993; Oldham et al., 1994; Hermann et al., 1994), Dufour’s gland (Hermann et al., 1994, Morgan et al., 2003), sting apparatus and venom gland (Hermann et al., 1994; Morgan et al., 2003), convoluted gland (inside venom reservoir; Schoeters & Billen, 1995), post-pharyngeal gland (Schoeters & Billen, 1997), and antennal sensillae and glands (Marques-Silva et al., 2006). Interestingly, Dinoponera lucida has the highest number of chromosomes known for any hymenopteran (2n=120), though the total number ranges from 2n=106 to 2n=120 and the significance or cause of this large number and variation is unknown (Mariano et al., 2004; Barros et al., 2009).
Lenhart et al. (2013) - Dinoponera is one of the roughly 10 ponerine genera in which some species have secondarily lost the typical morphologically specialized queen caste for a reproductive worker known as a gamergate (Haskins and Zahl 1971, Araujo et al. 1990, Paiva and Brandão 1995, Monnin and Peeters 1998, Peixoto et al. 2008). Conflict over dominance is intense in colonies with younger workers usually joining a linear hierarchy of one to five workers depending on colony size. The gamergate, or mated alpha worker has the highest rank (Monnin and Ratnieks 1999, Monnin et al. 2003). In Dinoponera quadriceps, the alpha worker mates with a non-nestmate male at night at the entrance of the nest (Monnin and Peeters 1998). After copulation the female bites through the male’s gaster to release herself and pulls out the genital capsule which acts as a temporary sperm plug (Monnin and Peeters 1998). After mating the female is unreceptive to other males and remains monandrous (Monnin and Peeters 1998). The gamergate maintains dominance with ritualized behaviors such as antennal boxing and biting, ‘blocking’, as well as gaster rubbing and curling (Monnin and Peeters 1999). Lipid stores within Dinoponera australis females were found to be strongly related to foraging activity and reproductive status within the colony, ranging from 1–39% of an individual’s dry mass (Smith et al. 2011). It is uncertain, however, whether nutritional differences between females is a cause or consequence of rank. Gamergate females possess a higher concentration of a cuticular hydrocarbon (9-hentriacontene, 9-C31:1) that indicates rank (Monnin et al. 1998, Peeters et al. 1999) and is passed onto gamergate-laid egg cuticles (Monnin and Peeters 1997). Additionally, alpha females may ‘sting smear’ a competing female with secretions from the Dufour’s gland, triggering the lower ranking workers to immobilize the marked female (Monnin and Ratnieks 2001). Subordinate females (beta, gamma, or delta) may produce unfertilized eggs but these are usually consumed by the alpha female in a form of “queen policing” (Monnin and Peeters 1997). Egg recognition in D. quadriceps was found to be due to differences in cuticular hydrocarbons, and only workers engaged in brood care could distinguish non-nestmate eggs (Tannure-Nascimento et al. 2009). Cuticular hydrocarbons are also used to distinguish adult nestmates from non-nestmates, however, this is only effective with non-nestmate foragers (Nascimento et al. 2012). Nascimento et al. (2012) found that brood-caring workers from different colonies had very similar hydrocarbon profiles and were more often accepted into alien colonies.
Males are born throughout most of the year in tropical species (Araujo and Jaisson 1994, Monnin and Peeters 1998), however D. australis which lives in the more temperate south was found to only produce males in May-July (Paiva and Brandão 1995). When the alpha declines reproductively or dies, she is replaced by a high-ranking worker (Monnin and Peeters 1999).
New colonies are founded by fission, a process in which a group of workers with brood leaves the natal nest, sometimes employing tandem running (Overal 1980). Colony fission creates an opportunity for a high-ranking worker to mate and become the new gamergate (Monnin and Peeters 1998).
Colonies vary in size depending upon species. Dinoponera australis colonies have an average of 14 workers (range 3–37) (Paiva and Brandão 1995, Monnin et al. 2003), D. gigantea average 41 workers (range ~30–96) (Overal 1980, Fourcassié and Oliviera 2002, Monnin et al. 2003) and D. quadriceps has the largest colonies with an average of 80 workers (range 26–238) (Monnin and Peeters 1999, Monnin and Ratnieks 2001). Morgan (1993) excavated two D. longipes nests, a possible incipient colony with 7 workers and another mature colony of 120 workers.
The nest consists of large chambers and tunnels in the soil possibly with an earthen mound and can be 0.10–1.2m deep (Araujo et al. 1990, Morgan 1993, Fourcassié and Oliviera 2002, Vasconcellos et al. 2004). Nests are deeper in D. australis and D. quadriceps than in D. gigantea, Monnin et al. (2003) suggests that deeper nests are a possible adaptation to seasons and aridity. Dinoponera gigantea nests may have up to eight entrances and can be weakly polydomous (Fourcassié and Oliviera 2002), whereas 1–30 openings with an average of 11 were recorded for D. longipes (Morgan 1993). Nesting density and spatial distribution varies depending on habitat (Fowler 1985, Vasconcellos et al. 2004). Density ranges from 15–40 nests per ha-1 (Vasconcellos et al. 2004) to 80 nests per ha-1 (Paiva and Brandão 1995). Morgan (1993) measured a spacing between nests for D. longipes with a median of 35m (n=22, range 14–69.5m). Dinoponera australis and D. gigantea usually nest at the base of trees (Paiva and Brandão 1995, Fourcassié and Oliviera 2002). Observations of D. quadriceps nests show that in more arid Caatinga and Cerrado habitats, nests are predominantly constructed under trees, whereas in Atlantic forest 60% of nests were 3m away from any tree (Vasconcellos et al. 2004).
Workers lower in the hierarchy forage individually for food items on the substrate and do not recruit other nestmates to assist with food transport (Fowler 1985, Fourcassié et al. 1999, Fourcassié and Oliviera 2002, Araújo and Rodrigues 2006). Although foraging workers do not recruit nestmates, Nascimento et al. (2012) found a positive feedback between incoming food and stimulation of new foragers as well as task partitioning once food was brought into the nest. Lower ranking females processed protein resources while higher ranking females handled small food pieces and distributed them to the larvae. Fourcassié and Oliviera (2002) found D. gigantea foraging to be concentrated in the early morning and afternoon but did not sample at night. Morgan (1993) observed the highest activity at night in D. longipes. Dinoponera quadriceps has a marked seasonal pattern in activity. It is most active in May-August, the late rainy season to early dry season in the semiarid Caatinga (Medeiros et al. 2012). Activity was strongly negatively correlated to temperature and positively correlated to prey abundance (Medeiros et al. 2012). The diets of both D. gigantea and D. quadriceps have been shown to be predominantly scavenged invertebrates, but include live prey, seeds and fruits (Zahl 1959, Fourcassié and Oliviera 2002, Araújo and Rodrigues 2006). Araújo and Rodrigues (2006) state that the taxonomic diversity of prey is comparable to other tropical ponerines, but has an optimal prey size of 2–3 cm in Dinoponera. Diet seems to be very similar across the genus, regardless of habitat (Araújo and Rodrigues 2006).
Despite their large size and strong venom, Dinoponera are likely preyed on by many vertebrate and invertebrate species across South America. Like many other ant species, Dinoponera can be infected by the entomopathogenic fungi Codyceps sp. (Evans 1982). Buys et al. (2010) discovered a Kapala sp. eucharitid wasp emerging from the puparia of Dinoponera lucida.
Anatomy has been described several times. Marques-Silva et al. (2006) studied of the sensilla and glands of the antennae. Anatomy of the venom apparatus and mandibular glands of Dinoponera gigantea is presented in Hermann et al. (1984). Further studies of the mandibular glands and its contents were presented by Oldham and Morgan (1993) and Oldham et al. (1994). Oldham et al. (1994) found that the mandibular gland secretions of workers differed greatly from those of gamergates, which were 98% dimethylalkylpyrazine and lacked the four other pyrazines and 50 times more volatiles than those found in worker secretions. The post-pharyngeal gland morphology was examined by Schoeters and Billen (1997). The cuticular hydrocarbons used in nestmate recognition may be produced by epidermal glands which Serrão et al. (2009) found in the epidermis of abdominal sternites in D. lucida.
For subduing large live prey and defense (Morgan, 1993), workers possess a sting that has been known to cause severe pain lasting up to 48 hours, lymphaedenopathy, edema, tachycardia and fresh blood to appear in human victim feces are common symptoms (Haddad et al. 2005). In gamergates the venom sac is empty (Monnin et al. 2002). Workers may have 60–75 unique proteinaceous components in the venom (Morgan et al. 2003, Johnson et al. 2010). The convoluted gland within the venom system of Dinoponera australis has been found to possess close similarities to those of vespine wasps (Schoeters and Billen 1995). The contents of D. australis venom have been found to be similar to those of Pachycondyla spp. (Cruz López 1994, Johnson et al. 2010). Billen et al. (1995) studied the morphology and ultrastructure of the pygidial gland of D. australis. Due to the high diversity of compounds and systemic effects found by Haddad et al. (2005), venom of Dinoponera could be of use to the pharmaceutical industry. For instance, Sousa et al. (2012) demonstrated in mice that venom from D. quadriceps had antinociceptive properties. The authors note that the local population of northeast Brazil uses dry crushed D. quadriceps ants to treat earaches, and the stings of live ants are administered for back pain and rheumatism.
Several studies of the cytogenetics of Dinoponera species have been conducted. Dinoponera lucida may have the highest number of chromosomes within the Hymenoptera however the karyotype is variable between populations (2n=106–120) (Mariano et al. 2004, Mariano et al. 2008, Barros et al. 2009). Mariano et al. (2008) interpreted the karyotype differences between populations as being due to a division of the species into allopatric populations during the Quaternary. Variability in the karyotype within a described species has been found in the Pachycondyla as well, and may represent cryp tic species (Mariano et al. 2012). Descriptions of the banding patterns on Dinoponera chromosomes are provided by Barros et al. (2009) and de Aguiar et al. (2011).
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Species Uncertain
- Unknown species are hosts for the phorid flies Apocephalus gigantivorus and Apocephalus miricauda (Brown et al., 2015).
- An unknown species is a host for the fungus Ophiocordyceps australis (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
All Associate Records for Genus
| Taxon | Relationship | Associate Type | Associate Taxon | Associate Relationship | Locality | Source | Notes |
|---|---|---|---|---|---|---|---|
| Dinoponera | host | fungus | Ophiocordyceps australis | parasitoid | Quevillon, 2018 | encounter mode primary; direct transmission; transmission outside nest | |
| Dinoponera | host | phorid fly | Apocephalus gigantivorus | parasite | Brown et al., 2015 | injured | |
| Dinoponera | host | phorid fly | Apocephalus miricauda | parasite | Brown et al., 2015 | injured | |
| Dinoponera gigantea | host | fungus | Ophiocordyceps myrmecophila | pathogen | Shrestha et al., 2017 | ||
| Dinoponera gigantea | host | phorid fly | Apocephalus gigantivorus | parasite | phorid.net | attacked | |
| Dinoponera gigantea | host | phorid fly | Apocephalus miricauda | parasite | phorid.net | attacked | |
| Dinoponera grandis | host | phorid fly | Apocephalus sp. | parasite | Brown et al., 2015 | injured | |
| Dinoponera longipes | associate (details unknown) | phorid fly | Metopina sp. | associate (details unknown) | Quevillon, 2018 | ||
| Dinoponera longipes | host | phorid fly | Apocephalus dinoponerae | parasite | Brown et al., 2015 | injured | |
| Dinoponera longipes | host | phorid fly | Apocephalus dinoponerae | parasite | phorid.net | attacked | |
| Dinoponera longipes | host | phorid fly | Apocephalus kungae | parasite | phorid.net | attacked | |
| Dinoponera longipes | host | phorid fly | Apocephalus kungae | parasite | Brown et al., 2015 | injured | |
| Dinoponera longipes | host | phorid fly | Apodicrania sp. | parasite | Brown et al., 2015 | injured | |
| Dinoponera longipes | host | phorid fly | Apodicrania sp. | parasitoid | Quevillon, 2018 | encounter mode primary; direct transmission; transmission outside nest | |
| Dinoponera longipes | host | phorid fly | Metopina-gp sp | parasite | Brown et al., 2015 | injured | |
| Dinoponera longipes | host | phorid fly | Rhyncophoromyia sp. | parasite | Brown et al., 2015 | injured | |
| Dinoponera longipes | host | phorid fly | Rhyncophoromyia sp. | parasitoid | Quevillon, 2018 | encounter mode primary; direct transmission; transmission outside nest | |
| Dinoponera lucida | host | eucharitid wasp | Kapala sp. | parasite | Universal Chalcidoidea Database | primary host | |
| Dinoponera lucida | host | phorid fly | Apocephalus exlucida | parasitoid | Quevillon, 2018 | encounter mode primary; direct transmission; transmission outside nest | |
| Dinoponera lucida | host | phorid fly | Megaselia sp. A | parasitoid | Quevillon, 2018 | encounter mode primary; direct transmission; transmission outside nest | |
| Dinoponera lucida | host | phorid fly | Megaselia sp. B | parasitoid | Quevillon, 2018 | encounter mode primary; direct transmission; transmission outside nest |
Life History Traits
- Queen type: gamergate (Peeters, 1997)
- Mean colony size: 30-60 (Greer et al., 2021)
- Compound colony type: not parasitic (Greer et al., 2021)
- Nest site: hypogaeic (Greer et al., 2021)
- Diet class: omnivore (Greer et al., 2021)
- Foraging stratum: subterranean/leaf litter (Greer et al., 2021)
- Foraging behaviour: solitary (Greer et al., 2021)
Castes
- Lenhart et al. (2013).
Morphology
Worker Morphology
 Explore: Show all Worker Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Worker Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
• Antennal segment count: 12 • Antennal club: absent • Palp formula: 4,4 • Total dental count: 6(1) • Spur formula: 2 (1 simple, 1 pectinate), 2 (1 simple, 1 pectinate) • Eyes: >100 ommatidia • Pronotal Spines: absent • Mesonotal Spines: absent • Propodeal Spines: absent • Petiolar Spines: absent • Caste: none or weak • Sting: present • Metaplural Gland: present • Cocoon: present
Male Morphology
 Explore: Show all Male Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Male Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
• Antennal segment count 13 • Antennal club 0 • Palp formula 5,3 • Total dental count 0 • Spur formula 2 pectinate, 2 pectinate;2 (1 barbulate, 1 pectinate), 2 (1 barbulate, 1 pectinate)
Karyotype
All Karyotype Records for Genus
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
| Taxon | Haploid | Diploid | Karyotype | Locality | Source | Notes |
|---|---|---|---|---|---|---|
| Dinoponera gigantea | 41 | 82 | Brazil | Santos et al., 2012; Mariano et al., 2015 | ||
| Dinoponera grandis | 57 | 114 | Brazil | Santos et al., 2012; Mariano et al., 2015 | as ''Dinoponera australis'' | |
| Dinoponera longipes | 31 | 62 | Brazil | Mariano et al., 2015 | ||
| Dinoponera lucida | 53 | 106 | 18M+88A | Brazil | Mariano et al., 2004a; Mariano et al., 2015 | Population polymorphism |
| Dinoponera lucida | 57 | 114 | Brazil | Mariano et al., 2008 | Population polymorphism | |
| Dinoponera lucida | 58 | 116 | Brazil | Mariano et al., 2008 | Population polymorphism | |
| Dinoponera lucida | 59 | 118 | Brazil | Mariano et al., 2008; Mariano et al., 2015 | Population polymorphism | |
| Dinoponera lucida | 59 | 118 | Brazil | Santos et al., 2012 | ||
| Dinoponera lucida | 60 | Brazil | Santos et al., 2012 | |||
| Dinoponera lucida | 60 | 120 | Brazil | Mariano et al., 2008; Mariano et al., 2015 | Population polymorphism | |
| Dinoponera quadriceps | 46 | 92 | Brazil | Santos et al., 2012; Mariano et al., 2015 |
Phylogeny
| Ponerinae |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See Phylogeny of Ponerinae for details.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- DINOPONERA [Ponerinae: Ponerini]
- Dinoponera Roger, 1861a: 37. Type-species: Ponera grandis (junior synonym of Ponera gigantea), by monotypy.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Schmidt and Shattuck (2014) - Huge (TL 25–40 mm; Kempf, 1971; Paiva & Brandão, 1995) ants with the standard characters of Ponerini. Mandibles subtriangular, with roughly five teeth and without a distinct basal angle or basal groove. Anterior margin of clypeus with a pair of long anteriorly-directed teeth. Frontal lobes moderately large. Eyes moderately large, located anterior of head midline and relatively forward-facing. Posterolateral corners of head prominent. Metanotal groove reduced to a subtle suture. Propodeum broad dorsally. Propodeal spiracles slit-like. Metapleural gland orifice with a posterior U-shaped cuticular lip and a lateral groove. Tarsal claws with a single tooth. Metatibial spur formula (1s, 1p). Petiole nodiform, with a blunt dorsal longitudinal ridge. Gaster with a strong girdling constriction between pre- and postsclerites of A4. Stridulitrum present on pretergite of A4. Hypopygium with a row of stout spines on either side of the sting. Head and body smooth to sparsely punctate, with sparse to abundant pilosity and patchy pubescence. Color black. See also description in Lenhart et al. (2013).
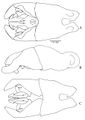
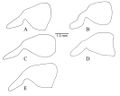
Lenhart et al. (2013) - Abundant setae; black integument, ranges from smooth and shiny with no microsculpturing, to finely micropunctate or scaled depending on species (Fig. 12). Head: Mandibles long and curved posteriorly in side view; seven large teeth; erect setae on dorsum. Ventral surface of head with sparse decumbent and subdecumbent setae; may have fine striations depending on species; Papal formula 4, 4; large bilobed labrum. Clypeus with two laterally projecting teeth on anterior edge, clypeus bulging medially, extending posteriorly between frontal lobes, anterior edge with row of long setae; sparse appressed setae from distal edges to medial area of clypeus. Area posterior to clypeus with varying amounts of striation. Tentorial pits apparent. Frontal lobes raised and conspicuous, with striations at posterior constriction. Antennae: geniculate, 12 segments, all with flagellate setae; scape long, extending past posterior border of head; funiculus covered in minute appressed pubescence. Gena depressed medially of eye; dense appressed setae on the antero-lateral sides of the head; covered in conflected punctulate sculpturing. Eyes large, elliptical with slight depression (ocular ring) around circumference. Frons with large pads of long flagellate pubescence (lost in older or poorly curated specimens). Median furrow running from posterior termination of clypeus, between frontal lobes to center of frons, terminates in shallow pit in most specimens. Entire head covered in long flagellate subdecumbent setae (Fig. 1A). Mesosoma: in lateral view weakly convex; covered in long subdecumbent to erect flagellate pilosity and dense pubescence; pronotal disc with slight bulges; promesonotal suture distinct, suture between mesopleuron and propodeum distinct; mesonotum fused with propodeum and episternum, separated by slight furrows; basilar sclerite large, ovaloid; propodeum with broadly rounded dorsal outline, dorsal surface gradually curves into posterior face (Fig. 2); propodeal spiracle forms nearly vertical slit; sulcus running from center of propodeum along lower edge of propodeal spiracle to posterior edge of propodeum at dorsal edge of bulla, patches of short white pubescence at curved posterior border of pronotum and basilar sclerite. Legs long, covered in long setae with short, stiff pubescence. One well-developed, antennae cleaning, comb-like spur on foreleg; one spine-like appendage and one less developed denticular comb on mesothoracic tibia; one spine and one comb-like spur on hind tibia. Posterior side of fore leg basitarsus with dense pads of golden setae; tarsal claws bidentate. Petiole: node large and tabular in lateral view, narrow attachments at base to propodeum and gaster; in dorsal view largest width less than propodeum and gaster, varies from ovate rectangular to ovate triangular in outline; covered in long subdecumbent to erect flagellate pilosity; pubescence on anterior face and ridges of subpetiolar process; subpetiolar process reduced, slightly variable between species. Gaster: typical of ponerines; covered with flagellate setae with short pubescence; small protuberance at articulation of gastric sternite III and the petiole; stridulatory file of varying size on acrotergite of gastral tergum II; posterior edges of the pygidium and hypopygidium with characteristic rows of minute spines.
Queen
Absent, reproduction instead being performed by gamergates.
Male
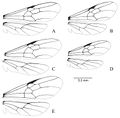
Lenhart et al. (2013) - Integument: smooth and nitid; reddish to dark brown/black. Head: Mandibles greatly reduced, rounded, spoon shaped, lacking teeth; palps elongated, maxillary palps 4 segmented, labial palps 3 segmented; labrum reduced, rounded to truncate, emarginated distal margin in D. snellingi and D. longipes covered with setae. Clypeus large, triangular, bulging medially; anterior tentorial pits large; frontal lobes absent; antennal sockets almost touching, located at posterior apex of clypeus. Antennae: geniculate, 13–segmented, pilosity varies from fine pubescence to long setae in different species; scape shorter than second funicular segment, but shorter than 1st, 1st funicular segment reduced. Compound eyes large, along lateral side of head, deeply emarginated medially. Three ocelli at posterior margin of head, bulging beyond margin of head in all species except D. australis. Entire head immaculate, covered in fine pubescence and long erect setae (Fig. 3). Mesosoma: pronotum triangular, exposed narrowly dorsally anterior to scutum; scutum large, bulging antero-dorsally, with 3 longitudinal carina; small tegula over insertion of forewing; scutellum domed, side with vertical carina, dorsal surface smooth; basilar sclerite under hind wing reduced; fused mesopleuron, separated by furrow into anepisternum and katepisternum; metanotum exposed between scutellum and propodeum, reduced; dorsal face of propodeum shorter than posterior face, rounded into posterior face; coxa large, conical (Fig. 3). Wings: covered in minute pubescence, venation as shown in Figure 5. Legs: one well-developed, antennae cleaning, pectinate spur on foreleg; one spine-like and one less developed denticular comb on mesothoracic tibia; one spine and one comblike spur on hind tibia. Posterior side of fore basitarsus with dense pads of golden setae; tarsal claws bidentate. Petiole: narrow attachments at base to propodeum and gaster; petiolar node humped dorsally, subpetiolar process anteriorly triangular. Gaster: large, cylindrical, covered in fine silvery pubescence; pygidium terminating in spine posteriorly, with short cerci; hypopygidium with long fine erect setae, tabular subgenital plate with posterior end truncated, often emarginated. Genitalia (Figs 6–11): basal ring with dorso-anterior loop structures; parameres long, rounded, with emarginated ventro-basal edge (Fig. 9); volsella articulated with basiparamere along ventral edge, lateral finger-like cuspis volsellaris, medial digitus volsellaris with distal wide toothed cusp, basal medial lobe with tooth-like structures varying with species (Fig. 10); penis valve of aedeagus roughly triangular and rounded, aedeagal apodeme curved horn-like antero-lateral arm structure arising from mid-valve ridge, terminating at interior surface of basiparamere (Fig. 11).
See also Tozetto & Lattke (2020).
Larva
Lenhart et al. (2013) - A basic description of the larva of D. quadriceps (cited as D. grandis mutica) is present in Mann (1916). A detailed description of the egg and all larval stages of Dinoponera gigantea are present in Wheeler and Wheeler (1985). The following generic description of Dinoponera larvae is from their work: “Profile pogonomyrmecoid (i.e., diameter greatest near the middle of abdomen, decreasing gradually toward anterior end and more rapidly toward posterior end, which is rounded; thorax more slender than abdomen and forming a neck, which is curved ventrally). Body with numerous (114–160) mammiform tubercles, each with 2–25 short simple hairs; body hairs lacking elsewhere. Cranial hairs lacking. Mandible dinoponeroid (i.e. narrowly subtriangular in anterior view; anterior portion curved posteriorly; with or without medial teeth.)”
References
- Ashmead, W. H. 1905c. A skeleton of a new arrangement of the families, subfamilies, tribes and genera of the ants, or the superfamily Formicoidea. Can. Entomol. 37: 381-384 (page 382, Dinoponera in Pachycondylinae, Ectatommini)
- Bolton, B. 2003. Synopsis and Classification of Formicidae. Mem. Am. Entomol. Inst. 71: 370pp (page 162, Dinoponera in Ponerinae, Ponerini)
- Boudinot, B.E. 2019. Hormigas de Colombia. Cap. 15. Clave para las subfamilias y generos basada en machos. Pp. 487-499 in: Fernández, F., Guerrero, R.J., Delsinne, T. (eds.) 2019d. Hormigas de Colombia. Bogotá: Universidad Nacional de Colombia, 1198 pp.
- Boudinot, B.E., Richter, A.K., Hammel, J.U., Szwedo, J., Bojarski, B., Perrichot, V. 2022. Genomic-phenomic reciprocal illumination: Desyopone hereon gen. et sp. nov., an exceptional Aneuretine-like fossil ant from Ethiopian amber (Hymenoptera: Formicidae: Ponerinae). Insects 13(9), 796 (doi:10.3390/insects13090796).
- Burchill, A.T., Moreau, C.S. 2016. Colony size evolution in ants: macroevolutionary trends. Insectes Sociaux 63, 291–298 (doi:10.1007/s00040-016-0465-3).
- Dalla Torre, K. W. von. 1893. Catalogus Hymenopterorum hucusque descriptorum systematicus et synonymicus. Vol. 7. Formicidae (Heterogyna). Leipzig: W. Engelmann, 289 pp. (page 31, Dinoponera in Ponerinae)
- Dias, A.M., Lattke, J.E. 2021. Large ants are not easy – the taxonomy of Dinoponera Roger (Hymenoptera: Formicidae: Ponerinae). European Journal of Taxonomy 784, 1–66 (doi:10.5852/ejt.2021.784.1603).
- Emery, C. 1895l. Die Gattung Dorylus Fab. und die systematische Eintheilung der Formiciden. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 8: 685-778 (page 767, Dinoponera in Ponerinae, Ponerini)
- Emery, C. 1911e. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125 (page 63, Dinoponera in Ponerinae, Ponerini [subtribe Pachycondylini])
- Esteves, F.A., Fisher, B.L. 2021. Corrieopone nouragues gen. nov., sp. nov., a new Ponerinae from French Guiana (Hymenoptera, Formicidae). ZooKeys 1074, 83–173 (doi:10.3897/zookeys.1074.75551).
- Fernandez, F., Guerrero, R.J., Sánchez-Restrepo, A.F. 2021. Sistemática y diversidad de las hormigas neotropicales. Revista Colombiana de Entomología 47, 1–20 (doi:10.25100/socolen.v47i1.11082).
- Forel, A. 1895b. A fauna das formigas do Brazil. Bol. Mus. Para. Hist. Nat. Ethnogr. 1: 89-139 (page 113, Dinoponera in Ponerinae, Ponerini)
- Forel, A. 1917. Cadre synoptique actuel de la faune universelle des fourmis. Bull. Soc. Vaudoise Sci. Nat. 51: 229-253 (page 237, Dinoponera in Ponerinae, Ponerini)
- Kempf, W. W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hymenoptera: Formicidae). Stud. Entomol. 14: 369-394 (page 369, Revision of genus)
- Lenhart, P.A., Dash, S.T. & Mackay, W.P. 2013. A revision of the giant Amazonian ants of the genus Dinoponera (Hymenoptera, Formicidae). Journal of Hymenoptera Research 31, 119–164.
- Mayr, G. 1862. Myrmecologische Studien. Verh. K-K. Zool.-Bot. Ges. Wien 12: 649-776 (page 714, Dinoponera in Ponerinae [Poneridae])
- Monnin T & Peeters C. 1997. Cannibalism of subordinates’ eggs in the monogynous queenless ant Dinoponera quadriceps. Naturwissenschaften 84: 499–502.
- Monnin T, Malosse C & Peeters C. 1998. Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in queenless ant Dinoponera quadriceps. Journal of Chemical Ecology 24:473–490.
- Monnin, T. & Peeters C. 1998. Monogyny and the regulation of worker mating in the queenless ant Dinoponera quadriceps. Animal Behaviour 55: 299-306.
- Monnin, T. & Peeters C. 1999. Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behavioral Ecology 10: 323-332.
- Peeters, C. 1997. Morphologically “primitive” ants: comparative review of social characters, and the importance of queen-worker dimorphism. Pages 372-391 In: Choe, J. & B. Crespi (eds) The Evolution of Social Behavior in Insects and Arachnids. Cambridge University Press. (doi:10.1017/CBO9780511721953.019).
- Roger, J. 1861a. Die Ponera-artigen Ameisen (Schluss). Berl. Entomol. Z. 5: 1-54 (page 37, Dinoponera as genus)
- Santos, I.S., Delabie, J.H.C., Silva, J.G., Costa, M.A., Barros, L.A.C., Pompolo, S.G. & Mariano, C.S.F. 2012. Karyotype differentiation among four Dinoponera (Formicidae: Ponerinae) species. Florida Entomologist 95(3), 737-742
- Schmidt, C.A. & Shattuck, S.O. 2014. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817, 1–242 (doi:10.11646/zootaxa.3817.1.1).
- Tozetto, L., Lattke, J.E. 2020. Revealing male genital morphology in the giant ant genus Dinoponera with geometric morphometrics. Arthropod Structure & Development 57, 100943 (doi:10.1016/j.asd.2020.100943)).
- Wheeler, W. M. 1910b. Ants: their structure, development and behavior. New York: Columbia University Press, xxv + 663 pp. (page 135, Dinoponera in Ponerinae, Ponerini)
- Wheeler, W. M. 1922i. Ants of the American Museum Congo expedition. A contribution to the myrmecology of Africa. VII. Keys to the genera and subgenera of ants. Bull. Am. Mus. Nat. Hist. 45: 631-710 (page 647, Dinoponera in Ponerinae, Ponerini)
- Pages using DynamicPageList3 parser function
- Articles using diversity taxobox
- Gamergate
- Photo Gallery
- Phorid fly Associate
- Host of Apocephalus gigantivorus
- Host of Apocephalus miricauda
- Fungus Associate
- Host of Ophiocordyceps australis
- Genus with Associate
- Genus with Karyotype
- Genus
- Extant genus
- Formicidae
- Ponerinae
- Ponerini
- Dinoponera
- Ponerinae genera
- Ponerini genera
- Gsr