Dinoponera gigantea
| Dinoponera gigantea | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Dinoponera |
| Species: | D. gigantea |
| Binomial name | |
| Dinoponera gigantea (Perty, 1833) | |
A soil nesting species with small colonies that average 41 workers.
| At a Glance | • Gamergate |
Identification
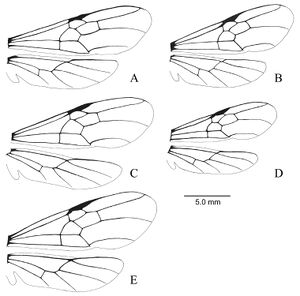
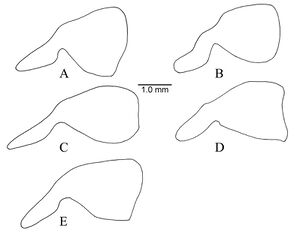
Lenhart et al. (2013) - Worker. Dinoponera gigantea can be distinguished from other Dinoponera species by the following combination of character states: antero-inferior corner of pronotum with distinct tooth-like process; integument finely micro-sculptured and not shiny; drab pilosity notably dense, long and flagellate; scape length longer than head width; total body length over 30 mm. Dinoponera gigantea is the largest species in the genus reaching up to 3.6 cm total body length. Dinoponera gigantea can be separated from all but two species by the presence of a tooth-like process on the antero-inferior corner of the pronotum. Dinoponera lucida and Dinoponera australis have a tooth-like process on the pronotum, but are smaller (usually less than 30 mm). In addition D. lucida has a shiny integument and D. australis lacks the long, flagellate pubescence.
Male. Males of this species are easily recognized by their funiculus which is covered in long standing setae, shiny dark reddish brown integument and the combination of a long pygidial spine, volsella with two basal teeth, lobed end of digitus volsellaris and deep concavity on the side of the penis valve of the aedeagus.
Keys
- Key to Dinoponera workers / Clave para la identificación de las obreras de Dinoponera / Chave para identificação de operários de Dinoponera
- Key to Dinoponera males / Clave para la identificación de los machos conocidos de Dinoponera / Chave para identificação de machos de Dinoponera
Keys including this Species
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: -0.690653° to -64.36°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil (type locality), Peru.
Dinoponera gigantea has been found on the coast of Guyana, in the Brazilian states of Amazonas, Pará including Marajo Island, Mato Grosso and Maranhão as well as the Loreto Province in Perú. Dinoponera gigantea is reported to be common in un-flooded forests in the vicinity of Belém, Pará (Kempf 1971, Overal 1980). It is probable that D. gigantea is found in French Guyana, Suriname, Venezuela and southeastern Colombia because these regions are adjacent to known D. gigantea localities and have similar lowland rainforest habitat. However, no specimens from these nations are known to us, perhaps as a result of a lack of sampling or the range is absent from Colombia and southwestern Venezuela.
A record from Rio de Janeiro (from the California Academy of Sciences) is puzzling as it is disjunct from the known range of D. gigantea. The most southeastern locality for D. gigantea is over 1,480km to the nearest portion of the state of Rio de Janeiro. In addition, Rio de Janeiro is in a well collected area where no other Dinoponera have been found. The label reads ‘R.d.Janeiro, Brazil, D. Davis’ and the specimen agrees in all morphological characters with D. gigantea. This locality is omitted from the species’ range map because of its questionable nature. If other collections can validate this locality it would mean a significant range extension for D. gigantea. (Lenhart, Dash & Mackay, 2013.)
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Diptera
- This species is a host for the phorid fly Apocephalus gigantivorus (a parasite) (phorid.net) (attacked).
- This species is a host for the phorid fly Apocephalus miricauda (a parasite) (phorid.net) (attacked).
Fungi
This species is a host for the fungus Ophiocordyceps myrmecophila (a pathogen) (Shrestha et al., 2017).
Castes
| |
| . | |
Image Gallery

| |
| . | |
Images from AntWeb
     
| |
| Male (alate). Specimen code casent0903662. Photographer Will Ericson, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- gigantea. Ponera gigantea Perty, 1833: 135, pl. 27, fig. 3 (w.) BRAZIL (Amazonas).
- Type-material: lectotype worker (by designation of Diller, 1990: 70), 2 paralectotype workers.
- Type-locality: lectotype Brazil: Rio Negro (“called Tucanquibura by locals”) (no collector’s name); paralectotypes with same data.
- Type-depository: ZSBS
- Kempf, 1971: 372 (m.).
- Combination in Dinoponera: Roger, 1861a: 38.
- Status as species: Borgmeier, 1937b: 225; Kempf, 1970b: 326; Kempf, 1971: 371 (redescription); Kempf, 1972a: 97; Bolton, 1995b: 171; Lenhart, et al. 2013: 139 (redescription); Bezděčková, et al. 2015: 123; Feitosa, 2015c: 98; Escárraga, et al. 2017: 134 (in key); Dias, A.M. & Lattke, 2021: 18 (redescription).
- Distribution: Brazil, Peru.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Lenhart et al. (2013) - (mm) (n=15) TBL: 31.62–36.02 (34.34); MDL: 4.59–5.35 (4.92); HL: 5.89–6.65 (6.31); HW: 5.74–6.27 (6.00); SL: 5.95–6.43 (6.30); WL: 8.71–9.94 (9.35); PL: 2.72–3.06 (2.81); PH: 3.08–3.67 (3.59); PW: 1.85–2.07 (1.98); GL: 9.43–12.24 (10.94); HFL: 8.10–9.3 (8.74).
A description of the external morphology of the worker is given in Kempf (1971): “Length of scape exceeding maximum width of head. Pubescence on front of head quite dense yet inconspicuous, not concealing the integument. Gular (ventral) surface of head reticulate-punctate throughout, very finely striate in front, the striae strongly converging mesad toward the anterior border. Sides of head reticulate-punctate, subopaque. Antero- inferior corner of pronotum dentate. Pronotal disc reticulate-punctate, subopaque, the paired swellings rather inconspicuous, but the median impression between swellings distinct, integument irregularly wrinkled. Tarsus I of hind leg longer than maximum head length. Petiole reticulate-punctate and subopaque, rectangular in profile, the anterior surface straight to slightly concave; the anterior upper corner more narrowly, the posterior corner more broadly rounded; posterior surface with the vertical sulcus always distinct; in dorsal view the petiole is decidedly longer than broad, width-length proportion below 0.80. Terga I and II of gaster opaque, sharply reticulate-punctate, densely foveolate (from each foveola arises a short decumbent hair), with scattered, bristle bearing, larger pints. The appressed pubescence, although inconspicuous, is densely spread over the entire terga, stridulatory file on acrotergite (portion of tergum that is normally concealed under the overlapping preceding tergum) of tergum II short, narrow, inconspicuous, not crossing beyond anterior half of acrotergite (hence not easily seen if entire acrotergite is not exposed).”
Male
Lenhart et al. (2013) - A description of the external morphology of the male is given in Kempf (1971). Measurements done by Kempf (1971) are included as only one male D. gigantea was examined by us while the measurements of Kempf (1971) most likely represent the means of the four males examined in that study: “Measurements in millimeters: total length 22.0; maximum length of head capsule 2.48; maximum width of head (eyes included) 3.10; maximum diameter of eyes 1.86; scape length 0.93; length of funicular articles I: 0.21, II: 1.86; Weber’s length of thorax 7.12; hind femur length 5.57; hind tarsus I length 5.38; petiole length 2.16, width 1.24, height 1.76; tergum I of gaster length 3.09, width 2.88; fore wing length 15.6; hind wing length 12.15. Chestnut brown, smooth and shining except funiculi, clypeus, front, tibiae and tarsi which are finely punctuate to reticulate-punctate; terga III and following of gaster weakly, superficially and finely reticulate. The entire insect covered with long, subdecumbent, silky pubescence, except funiculi where the pubescence is minute. Standing hairs long and abundant on body, lacking on mid-dorsum of terga II-V of gaster; long hairs on scapes rather numerous, length not much longer than twice the diameter of scape…Anterior border of labrum rounded, not visibly excised… Pygidial spine long and well developed. Parameres (gonostyli) of genitalia in side-view narrow and spear-pointed… Hypopygidium (subgenital plate of subandrium) apically rounded…”
Genitalia. Basal ring with wide dorso-anterior loop structures, dorsal depression on basal ring posterior to cleft between dorso-anterior loops, ridge extending from anterior of depression to center; parameres long, narrow, rounded spade-shape, emarginated ventro-basal edge; volsella with finger-like cuspis volsellaris and broad cusp-like digitus volsellaris, cuspis volsellaris with pointed end, flange extending on dorsal edge, digitus volsellaris with numerous small circular bumps, lobe on postero-dorsal edge, 2 teeth on medial ventro-basal corner of volsella, posterior tooth with lobe on posterior edge; penis valve of aedeagus with lateral arm of apodeme at anterior border, deep, wide, ventral concavity under ridge at base of apodeme, distal edge of valve wedge-shaped, proximal ventral edge of valve ending in tooth descending at roughly 45°, ventral edge with large laterally curved lip, serrated edge, serrations facing laterally on either side of aedeagus in dorsal view.
See also Tozetto & Lattke (2020).
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- n = 41, 2n = 82 (Brazil) (Santos et al., 2012; Mariano et al., 2015).
References
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Camargo, K.S. de. 2011. Composicao e diversidade de "Poneromorfas" (Hymenoptera, Formicidae) em duas fitofisionomias de cerrado e padroes de distribuicao de "Poneromorfas", Pseudomyrmecinae e Cephalotini (Myrmicinae) para o Brasil. Thesis, Universidade de Brasilia.
- Casadei-Ferreira, A., Fischer, G., Economo, E.P. 2020. Evidence for a thoracic crop in the workers of some Neotropical Pheidole species (Formicidae: Myrmicinae). Arthropod Structure, Development 59, 100977 (doi:10.1016/J.ASD.2020.100977).
- Curbani, F., Zocca, C., Ferreira, R.B., Waichert, C., Sobrinho, T.G., Srbek-Araujo, A.C. 2021. Litter Surface Temperature: A Driving Factor Affecting Foraging Activity in Dinoponera lucida (Hymenoptera: Formicidae). Sociobiology 68: e-6030 (doi:10.13102/sociobiology.v68i1.6030).
- de Aguiar HJAC, Barros LAC, dos Santos Ferreira Mariano C, Delabie JHC and das Gracas Pompolo S. 2011. 45S rDNA localization for the giant ant Dinoponera gigantea with evolutionary inferences for the Dinoponera genus (Formicidae: Ponerinae). Sociobiology 57:607.
- Dias, A.M., Lattke, J.E. 2021. Large ants are not easy – the taxonomy of Dinoponera Roger (Hymenoptera: Formicidae: Ponerinae). European Journal of Taxonomy 784, 1–66 (doi:10.5852/ejt.2021.784.1603).
- Fourcassié V and Oliveira PS. 2002. Foraging ecology of the giant Amazonian ant Dinoponera gigantea (Hymenoptera, Formicidae, Ponerinae): activity schedule, diet and spatial foraging patterns. Journal of Natural History 36: 2211–2227. doi: 10.1080/00222930110097149
- Fourcassié V, Henriques A and Fontella C. 1999. Route fidelity and spatial orientation in the ant Dinoponera gigantea (Hymenoptera, Formicidae) in a primary forest: a preliminary study. Sociobiology 34:505–524.
- Guénard, B., Silverman, J. 2011. Tandem carrying, a new foraging strategy in ants: description, function, and adaptive significance relative to other described foraging strategies. Naturwissenschaften 98(8), 651–659 (doi:10.1007/s00114-011-0814-z).
- Haddad Junior V, Cardoso JLC and Moraes RHP. 2005. Description of an injury in a human caused by a false tocandira (Dinoponera gigantea, Perty, 1833) with a revision on folkloric, pharmacological and clinical aspects of the giant ants of the genera Paraponera and Dinoponera (family Ponerinae). Revista do Instituto de Medicina Tropical de São Paulo 47: 235–238. doi: 10.1590/S0036-46652005000400012
- Haskins, C.P. & Zahl, P.A. (1971) The reproductive pattern of Dinoponera grandis Roger (Hymenoptera, Ponerinae) with notes on the ethology of the species. Psyche (Camb.), 78 (1–2), 1–11.
- Hermann, H. R.; Blum, M. S.; Wheeler, J. W.; Overal, W. L.; Schmidt, J. O.; Chao, J. 1984. Comparative anatomy and chemistry of the venom apparatus and mandibular glands in Dinoponera grandis (Guérin) and Paraponera clavata (F.) (Hymenoptera: Formicidae: Ponerinae). Ann. Entomol. Soc. Am. 77: 272-279 PDF
- Kempf, W. W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hymenoptera: Formicidae). Stud. Entomol. 14: 369-394 (page 372, male described; page 371, Senior synonym of grandis)
- Lenhart, P.A., Dash, S.T. & Mackay, W.P. 2013. A revision of the giant Amazonian ants of the genus Dinoponera (Hymenoptera, Formicidae). Journal of Hymenoptera Research 31, 119–164.
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Overal WL. 1980. Observations on colony founding and migration of Dinoponera gigantea. Journal of the Georgia Entomological Society 15:466–469.
- Perty, M. 1833. Delectus animalium articulatorum, quae in itinere per Brasiliam annis MDCCCXVII-MDCCCXX jussu et auspiciis Maximiliani Josephi I. Bavariae regis augustissimi peracto, collegerunt Dr. J. B. Spix et Dr. C. F. Ph. de Martius. Fasc. 3. Monach (page 135, pl. 27, fig. 3 worker described)
- Roger, J. 1861a. Die Ponera-artigen Ameisen (Schluss). Berl. Entomol. Z. 5: 1-54 (page 38, Combination in Dinoponera, Senior synonym of grandis)
- Santos, I.S., Delabie, J.H.C., Silva, J.G., Costa, M.A., Barros, L.A.C., Pompolo, S.G. & Mariano, C.S.F. 2012. Karyotype differentiation among four Dinoponera (Formicidae: Ponerinae) species. Florida Entomologist 95(3), 737-742
- Shrestha B, Tanaka E, Hyun MW, Han JG, Kim CS, Jo JW, Han SK, Oh J, Sung JM, Sung GH. 2017. Mycosphere Essay 19. Cordyceps species parasitizing hymenopteran and hemipteran insects. Mycosphere 8(9): 1424–1442 (DOI 10.5943/mycosphere/8/9/8).
- Tozetto, L., Lattke, J.E. 2020. Revealing male genital morphology in the giant ant genus Dinoponera with geometric morphometrics. Arthropod Structure & Development 57, 100943 (doi:10.1016/j.asd.2020.100943)).
- Zocca, C., Curbani, F., Ferreira, R.B., Weichert, C., Sobrinho, T.G., Srbek-Araujo, A.C. 2021. A day in the life of the giant ant Dinoponera lucida Emery, 1901 (Hymenoptera, Formicidae): Records of activities and intraspecific interactions. Sociobiology 68, 6166 (doi:10.13102/sociobiology.v68i2.6166).
References based on Global Ant Biodiversity Informatics
- Borgmeier T. 1923. Catalogo systematico e synonymico das formigas do Brasil. 1 parte. Subfam. Dorylinae, Cerapachyinae, Ponerinae, Dolichoderinae. Archivos do Museu Nacional (Rio de Janeiro) 24: 33-103.
- Emery C. 1896. Formiciden, gesammelt in Paraguay von Dr. J. Bohls. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 9: 625-638.
- Escalante Gutiérrez J. A. 1993. Especies de hormigas conocidas del Perú (Hymenoptera: Formicidae). Revista Peruana de Entomología 34:1-13.
- Fernandes I., and J. de Souza. 2018. Dataset of long-term monitoring of ground-dwelling ants (Hymenoptera: Formicidae) in the influence areas of a hydroelectric power plant on the Madeira River in the Amazon Basin. Biodiversity Data Journal 6: e24375.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Forel A. 1909. Ameisen aus Guatemala usw., Paraguay und Argentinien (Hym.). Deutsche Entomologische Zeitschrift 1909: 239-269.
- Kempf W. W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hymenoptera: Formicidae). Studia Entomologica 14: 369-394.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Lenhart, P. A.; Dash, S. T.; and Mackay, W. P. 2013. A revision of the giant Amazonian ants of the genus Dinoponera (Hymenoptera, Formicidae). Journal of Hymenoptera Research 31: 119-164
- Luederwaldt H. 1918. Notas myrmecologicas. Rev. Mus. Paul. 10: 29-64.
- Monnin, R., F.L.W. Ratnieks and C.R.F. Brandao. 2003. Reproductive Conflict in Animal Societies: Hierarchy Length Increases with Colony Size in Queenless Ponerine Ants. Behavioral Ecology and Sociobiology 54(1):71-79
- Pires de Prado L., R. M. Feitosa, S. Pinzon Triana, J. A. Munoz Gutierrez, G. X. Rousseau, R. Alves Silva, G. M. Siqueira, C. L. Caldas dos Santos, F. Veras Silva, T. Sanches Ranzani da Silva, A. Casadei-Ferreira, R. Rosa da Silva, and J. Andrade-Silva. 2019. An overview of the ant fauna (Hymenoptera: Formicidae) of the state of Maranhao, Brazil. Pap. Avulsos Zool. 59: e20195938.
- Santschi F. 1939. Résultats scientifiques des croisières du navire-école belge, "Mercator". XIV. Formicidae s. lt. Mémoires du Musée Royal d'Histoire Naturelle de Belgique. (2)15: 159-167.
- Pages using DynamicPageList3 parser function
- Gamergate
- Tropical
- South subtropical
- South temperate
- Phorid fly Associate
- Host of Apocephalus gigantivorus
- Host of Apocephalus miricauda
- Fungus Associate
- Host of Ophiocordyceps myrmecophila
- Karyotype
- Species
- Extant species
- Formicidae
- Ponerinae
- Ponerini
- Dinoponera
- Dinoponera gigantea
- Ponerinae species
- Ponerini species
- Dinoponera species
- Ssr

