Dinoponera quadriceps
| Dinoponera quadriceps | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Dinoponera |
| Species: | D. quadriceps |
| Binomial name | |
| Dinoponera quadriceps Kempf, 1971 | |
| Synonyms | |
| |
A queenless species with small colonies (average 80 workers) nesting underground. This is the highest average colony size of all Dinoponera studied.
| At a Glance | • Gamergate |
Identification
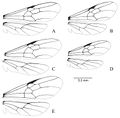
Lenhart et al. (2013) - Workers are recognized by finely micro-sculptured integument which is not shiny, rounded anterior inferior pronotal corner lacking a tooth-like process, ventral side of the head lacking any gular striations and long/flagellate pilosity.
Male. Males are distinguished by the long fine setae of the second funicular segment, light brown coloration, long narrow parameres, volsella with two small basal teeth and lacking a lobe on the distal edge of digitus volsellaris.
Dinoponera quadriceps may be confused with Dinoponera mutica, but has a finely micro-sculptured integument which is not shiny, lacks gular striations and has a petiole which bulges on the dorso-anterior edge in contrast to D. mutica’s roughly microsculptured integument, striated gula and petiole with even, non-bulging corners.
- Lenhart et al. (2013).
Keys
- Key to Dinoponera workers / Clave para la identificación de las obreras de Dinoponera / Chave para identificação de operários de Dinoponera
- Key to Dinoponera males / Clave para la identificación de los machos conocidos de Dinoponera / Chave para identificação de machos de Dinoponera
Keys including this Species
Distribution
Dinoponera quadriceps is found in the Caatingas, Cerrados, upland humid forest and Atlantic forest (Kempf 1971, Paiva and Brandão 1995) in the northeastern Brazilian states of Alagoas, Bahia, Ceará, Paraiba, Pernambuco and Rio Grande do Norte (Lenhart, Dash & Mackay, 2013).
Latitudinal Distribution Pattern
Latitudinal Range: -3.3247° to -27.352778°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Patalano et al. (2015) conducted a ground breaking study of genetic differences among castes in Dinoponera quadriceps and the wasp Polistes canadensis. The work sought to understand the genetic control of phenotypic plasticity.
"The two study species (the dinosaur ant Dinoponera quadriceps and the paper wasp Polistes canadensis) exhibit very simple societies, where individuals retain the ability to switch phenotype. This characteristic contrasts with the adult honey bee Apis mellifera and most ants, which exhibit low levels of phenotypic plasticity and have been the focus of most previous molecular analyses. Our two study species share similar levels of plasticity among individuals, with a single reproductive egg-layer (“gamergate” in D. quadriceps and “queen” in P. canadensis) that is morphologically identical to the nonreproductives; if the reproductive dies, it is quickly replaced by one of the nonreproductives. Both species share many ecological traits but evolved social phenotypes independently.
We sequenced the genomes, miRNAs, multiple brain transcriptomes, and methylomes from two eusocial insect species whose life cycles depend on high phenotypic plasticity throughout life. This data includes the first aculeate wasp genome sequence to our knowledge. Both species displayed three key molecular signatures that may be molecular hallmarks for highly plastic phenotypes in simple eusocial insects. These key molecular signatures are as follows: (i) little molecular differentiation between phenotypes in transcription but subtle nonrandom differentiation at the transcriptional network level; (ii) no evidence of a role for DNA methylation or miRNAs in regulating phenotypic differentiation and an overall lack of distinct methylome patterning, together with evidence of methylation turnover; and (iii) a similar role for both conserved toolkit genes and previously unidentified taxonomically restricted genes in phenotypic differentiation. These characteristics may allow plasticity in the regulation of the genome, and thus facilitate plasticity at the phenotypic level (52). The sequencing of more species with different levels of plasticity and multiple phenotypes will be required to confirm this hypothesis (6). However, the available data suggest that these hallmarks contrast with those hallmarks of eusocial insects with low plasticity like the honey bee and most ants, where a large proportion of genes, functionality, and network differentiation are associated with phenotypic differentiation, and where phenotypes appear to be regulated by DNA methylation."
Within its range Dinoponera quadriceps is used locally for medicinal uses. Medeira et al (2015) studied the venom for its effectiveness in mitigating a number of human ills. Their abstract: The South American giant ant, Dinoponera quadriceps (Hymenoptera, Formicidae, Ponerinae), produces proteinaceous venom that has antinociceptive, neuroprotective and antimicrobial effects, thereby supporting the popular use of these ants to treat asthma, rheumatism, earache and back pain. Anticoagulant activity is another biological property that has been shown for the venom of other hymenopteran species, like wasps. The aim of this study was to assess the anti-inflammatory, anticoagulant and antiplatelet properties of D. quadriceps venom (DqV). DqV anti-inflammatory activity was assessed by intravenous administration in Swiss mice in the models of paw edema and peritonitis. In vitro, DqV was assessed in coagulation (activated partial thromboplastin time) and platelet aggregation tests. DqV inhibited (27-33%) the edema elicited by carrageenan and the leucocyte migration (43%) elicited by zymosan. DqV decreased by 57% and 42%, respectively, the content of malondialdehyde and nitrite in the peritoneal fluid. DqV prolonged (1.8x) the clotting time and decreased (27%) the platelet aggregation induced by adenosine diphosphate. The crude venom of D. quadriceps presents an anti-inflammatory effect in mice and in vitro anticoagulant and antiplatelet effects.
Life History Traits
- Mean colony size: 60 (Beckers et al., 1989)
- Foraging behaviour: solitary forager (Beckers et al., 1989)
Castes
Queen caste absent, which is a secondary evolutionary modification characteristic of the genus. All workers are morphologically similar and able to reproduce sexually (Monnin & Peeters 1998). A dominance hierarchy is established among young workers, and only the alpha worker is able to mate with a foreign male (thus becoming the gamergate). Low-ranking workers also participate in the behavioural regulation ('policing') of monogyny (Monnin & Peeters 1999).
Dinoponera X-ray micro-CT scan 3D model of Dinoponera quadriceps (worker) prepared by the Economo lab at OIST.
X-ray micro-CT scan 3D model of Dinoponera quadriceps (worker) prepared by the Economo lab at OIST.
See on Sketchfab. See list of 3D images.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- quadriceps. Dinoponera quadriceps Kempf, 1971: 380.
- Type-material: holotype worker.
- Type-locality: Brazil: Pernambuco, São Lourenço da Mata, Tapera (Reichensperger).
- Type-depository: NHMB.
- [First available use of Dinoponera grandis st. mutica var. quadriceps Santschi, 1921g: 84 (w.) BRAZIL (no state data); unavailable (infrasubspecific) name.]
- Borgmeier, 1937b: 227 (m.).
- As unavailable (infrasubspecific) name: Borgmeier, 1923: 102; Borgmeier, 1937b: 226.
- Status as species: Kempf, 1972a: 97; Kempf, 1975c: 344; Brandão, 1991: 340; Bolton, 1995b: 171; Lenhart, et al. 2013: 149 (redescription); Bezděčková, et al. 2015: 123; Feitosa, 2015c: 98; Escárraga, et al. 2017: 134 (in key); Dias, A.M. & Lattke, 2021: 50 (redescription).
- Senior synonym of opaca: Kempf, 1975c: 344; Brandão, 1991: 340; Bolton, 1995b: 171; Lenhart, et al. 2013: 149; Dias, A.M. & Lattke, 2021: 50.
- Distribution: Brazil.
- opaca. Dinoponera opaca Kempf, 1971: 379.
- Type-material: holotype worker.
- Type-locality: Brazil: Rio de Janeiro (Goeldi).
- Type-depository: NHMB.
- [First available use of Dinoponera grandis st. mutica var. opaca Santschi, 1921g: 84 (w.) BRAZIL (Rio de Janeiro); unavailable (infrasubspecific) name.]
- As unavailable (infrasubspecific) name: Borgmeier, 1923: 102; Borgmeier, 1937b: 225.
- Status as species: Kempf, 1972a: 97.
- Junior synonym of quadriceps: Kempf, 1975c: 344; Brandão, 1991: 340; Bolton, 1995b: 171; Lenhart, et al. 2013: 149; Dias, A.M. & Lattke, 2021: 50.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Lenhart et al. (2013) - (mm) (n=17) TBL: 28.09–33.73 (30.60); MDL: 4.10–5.05 (4.53); HL: 5.23–6.04 (5.58); HW: 5.33–5.97 (5.56); SL: 5.54–6.12 (5.80); WL: 7.38–9.03 (8.20); PL: 2.26–2.68 (2.50); PH: 3.06–3.52 (3.26); PW: 1.64–1.99 (1.80); GL: 8.20–11.93 (9.80); HFL: 7.18–8.11 (7.65).
A description of the worker is given in Emery 1911, Mann 1916, Borgmeier 1937, Kempf 1971. Presented below is that of Kempf (1971): “Antennal scape notably longer than head width. Pubescence on front and vertex of head short and inconspicuous. Gular surface of head reticulate-punctate, subopaque, but lacking arcuate striae except for some cases when a few short and vestigial striae appear antero-laterally, just behind the mandibular insertion. Sides of head reticulate-punctate, subopaque. Antero-lateral corner of pronotum obtusely angulate (very seldom subdentate). Pronotal disc reticulate-punctate, subopaque, occasionally slightly wrinkled, bristle pits irregular in outline; paired swellings very faint and obsolete. Hind tarsus I longer than head length. Petiole… of distinctive shape, the anterior surface being slightly inclined forward and often a bit excavate; anterior upper corner narrowly, the posterior corner very broadly rounded; integument minutely reticulate-punctate and subopaque; sulcus on posterior surface always distinct. Terga I and II of gaster reticulate-punctate and opaque; piligerous pits for pubescence discally greatly scattered (in a few southern specimens from Bahia State, these pits are stronger and denser, almost as in gigantea); coarse bristle-bearing pits greatly scattered: pubescence rather scarce on dorsum, denser and more conspicuous on sides. Stridulatory file on tergum II of gaster weakly developed, arising from the anterior border of acrotergite and running streak-like across the anterior half of the same (visible only when acrotergite is fully exposed; observed in five specimens).”
Male
Total length 21mm (Mann 1916) 22 mm (Borgmeier 1937).
A description of the male is given in Emery (1911), Mann (1916), Borgmeier (1937), and Kempf (1971). Mann (1916) described the male as follows: “Head, including the mandibles, as broad as long, very convex behind. Eyes very large and long occupying the entire sides of head, the inner border deeply emarginate; ocelli very large and convex. Clypeus convex, the anterior border truncate. Mandibles small, pointed at apex, with a small tooth at middle of inner border. Antennae a little shorter than the body; first funicular joint twice as broad as long; joints 2–11 very long, cylindrical, each slightly shorter and more slender than the preceding. Thorax [= mesosoma] robust; scutellum short, triangular, broadly rounded at apex. Epinotum [= propodeum] evenly rounded, without distinct base or declivity, unarmed. Petiole nearly twice as long as broad, narrowed in front, with nearly straight sides; in profile longer than high, flattened above…the anterior slop gradual, more abruptly sloping behind, the antero-ventral surface with a broad, triangular projection. Gaster long and slender, the three times the breadth. Genitalia prominent; the valves board, rounded at apex; cerci long and slender…Wings large extending almost to the tip of gaster … Legs very long and slender…Body and legs shining. Antennae opaque, coarsely, densely punctured; sparsely pubescent, and having much very long, fine erect hairs, which on the apical joints are shorter and confined to the tips; pubescence of apical joint more dense than the rest. Thorax (=mesosoma ) with long silky pubescence, most abundant on the pleurae, and very fine re erect hairs sparsely distributed node without pubescence, but with abundant erect hairs. Gaster with a thick mat of silky pubescence, shorter and finer than that of the thorax (=mesosoma); lateral and apical portions with fine erect hairs… Color rufous, the antennal scape and the first five funicular joints fucous. Wings lightly infuscated, veins and stigma reddish brown. Pubescence yellowish white, exempt the long antennal hairs which are black.”
To this Borgmeier (1937) added that the petiole was “rounded on top”, “the sting of the pygidium [=pygidial spine] long; subgential plate with apex slightly concave”, and that the wings were 16mm long and “slightly yellowish”. Kempf (1971) noted the dorsum of the gaster lacked standing hairs.
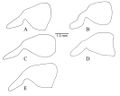
Genitalia. Basal ring with wide, thin dorso-anterior loop structures; parameres distinctly long, narrow, rounded end, emarginated ventro-basal edge; cuspis volsellaris finger-like with few rounded bumps on medial face, digitus volsellaris broad cusp-like with numerous small circular bumps, 2 teeth at ventro-basal corner of volsella; penis valve of aedeagus with lateral arm of apodeme at anterior border, slight ventral concavity under ridge at base of apodeme, distal edge wedge-shaped, proximal ventral edge of penal valve ending in anterior facing tooth, ventral edge with large dorso-laterally curved lip with serrated edge, serrations facing laterally on either side of dorsally curved lip.
See also Tozetto & Lattke (2020).
Karyotype
- n = 46, 2n = 92 (Brazil) (Santos et al., 2012; Mariano et al., 2015).
References
- Medeiros Jennifer, Arrilton Araujo, Helder F. P. Araújo, João Paulo C. Queiroz, Alexandre Vasconcellos. 2012. Seasonal activity of Dinoponera quadriceps Santschi (Formicidae, Ponerinae) in the semi-arid Caatinga of northeastern Brazil. Revista Brasileira de Entomologia 56(1):81-85.
- Addo-Bediako, A., Chown, S.L., Gaston, K.J. 2001. Revisiting water loss in insects: a large scale view. Journal of Insect Physiology 47, 1377–1388 (doi:10.1016/s0022-1910(01)00128-7).
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Araujo CZ and Jaisson P. 1994. Modes de fondation des colonies chez la fourmi sans reine Dinoponera quadriceps Santschi (Hymenoptera, Formicidae, Ponerinae). Actes des Colloques Insectes Sociaux 9:79–88.
- Araújo, A., Rodriques, Z. 2006. Foraging behavior of the queenless ant Dinoponera quadriceps Santschi (Hymenoptera: Formicidae). Neotropical Entomology 35:159–164 (DOI 10.1590/S1519-566X2006000200002).
- Araujo, E.S., Koch, E.B.A., Delabie, J.H.C., Zeppelini, D., DaRocha, W.D., Castaño-Meneses, G., Mariano, C.S.F. 2019. Diversity of commensals within nests of ants of the genus Neoponera (Hymenoptera: Formicidae: Ponerinae) in Bahia, Brazil. Annales de la Société entomologique de France (N.S.), 1–9. (doi:10.1080/00379271.2019.1629837).
- Azevedo, D.L.O., Medeiros, J.da C., Araújo, A. 2021. Flexibility in the integration of environmental information by Dinoponera quadriceps Kempf during foraging. Revista Brasileira de Entomologia 65, e20210084 (doi:10.1590/1806-9665-rbent-2021-0084).
- Baer, B. 2011. The copulation biology of ants (Hymenoptera: Formicidae). Myrmecological News 14: 55-68.
- Beckers R., Goss, S., Deneubourg, J.L., Pasteels, J.M. 1989. Colony size, communication and ant foraging Strategy. Psyche 96: 239-256 (doi:10.1155/1989/94279).
- Billen, J.P.J. 2019. Diversidad y morfología de las glándulas exocrinas en las hormigas. Pp. 165-174 in: Fernández, F., Guerrero, R.J., Delsinne, T. (eds.) 2019d. Hormigas de Colombia. Bogotá: Universidad Nacional de Colombia, 1198 pp.
- Camargo, K.S. de. 2011. Composicao e diversidade de "Poneromorfas" (Hymenoptera, Formicidae) em duas fitofisionomias de cerrado e padroes de distribuicao de "Poneromorfas", Pseudomyrmecinae e Cephalotini (Myrmicinae) para o Brasil. Thesis, Universidade de Brasilia.
- Cronin, A.L., Monnin, T. 2009. Bourgeois queens and high stakes games in the ant Aphaenogaster senilis. Frontiers in Zoology 6, 24. (doi:10.1186/1742-9994-6-24).
- Curbani, F., Zocca, C., Ferreira, R.B., Waichert, C., Sobrinho, T.G., Srbek-Araujo, A.C. 2021. Litter Surface Temperature: A Driving Factor Affecting Foraging Activity in Dinoponera lucida (Hymenoptera: Formicidae). Sociobiology 68: e-6030 (doi:10.13102/sociobiology.v68i1.6030).
- Cuvillier-Hot, V., Lenoir, A. 2006. Biogenic amine levels, reproduction and social dominance in the queenless ant Streblognathus peetersi. Naturwissenschaften 93, 149–153 (doi:10.1007/s00114-006-0086-1).
- Dias, A.M., Lattke, J.E. 2021. Large ants are not easy – the taxonomy of Dinoponera Roger (Hymenoptera: Formicidae: Ponerinae). European Journal of Taxonomy 784, 1–66 (doi:10.5852/ejt.2021.784.1603).
- Esteves, F.A., Fisher, B.L. 2021. Corrieopone nouragues gen. nov., sp. nov., a new Ponerinae from French Guiana (Hymenoptera, Formicidae). ZooKeys 1074, 83–173 (doi:10.3897/zookeys.1074.75551).
- Gospocic, J., Glastad, K.M., Sheng, L., Shields, E.J., Berger, S.L., Bonasio, R. 2021. Kr-h1 maintains distinct caste-specific neurotranscriptomes in response to socially regulated hormones. Cell 184, 5807–5823.e14 (doi:10.1016/j.cell.2021.10.006).
- Guénard, B., Silverman, J. 2011. Tandem carrying, a new foraging strategy in ants: description, function, and adaptive significance relative to other described foraging strategies. Naturwissenschaften 98(8), 651–659 (doi:10.1007/s00114-011-0814-z).
- Jacobs, S. 2020. Population genetic and behavioral aspects of male mating monopolies in Cardiocondyla venustula (Ph.D. thesis).
- Kempf, W. W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hymenoptera: Formicidae). Stud. Entomol. 14: 369-394 (page 380, worker described)
- Kempf, W. W. 1975c. Miscellaneous studies on neotropical ants. VI. (Hymenoptera, Formicidae). Stud. Entomol. 18: 341-380 (page 344, Senior synonym of opaca)
- Lau, M.K., Ellison, A.M., Nguyen, A., Penick, C., DeMarco, B., Gotelli, N.J., Sanders, N.J., Dunn, R.R., Helms Cahan, S. 2019. Draft Aphaenogaster genomes expand our view of ant genome size variation across climate gradients. PeerJ 7, e6447 (doi:10.7717/PEERJ.6447).
- Lenhart, P.A., Dash, S.T. & Mackay, W.P. 2013. A revision of the giant Amazonian ants of the genus Dinoponera (Hymenoptera, Formicidae). Journal of Hymenoptera Research 31, 119–164.
- Madeira, J. D., Y. P. Quinet, D. T. T. Nonato, P. L. Sousa, E. M. C. Chaves, J. E. R. Honorio, M. G. Pereira, and A. M. S. Assreuy. 2015. Novel Pharmacological Properties of Dinoponera quadriceps Giant Ant Venom. Natural Product Communications. 10:1607-1609.
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Monnin T & Peeters C. 1997. Cannibalism of subordinates’ eggs in the monogynous queenless ant Dinoponera quadriceps. Naturwissenschaften 84: 499–502.
- Monnin T, Malosse C & Peeters C. 1998. Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in queenless ant Dinoponera quadriceps. Journal of Chemical Ecology 24:473–490.
- Monnin, T. & Peeters C. 1998. Monogyny and the regulation of worker mating in the queenless ant Dinoponera quadriceps. Animal Behaviour 55: 299-306.
- Monnin, T. & Peeters C. 1999. Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behavioral Ecology 10: 323-332.
- Nagel, M., Qiu, B., Brandenborg, L.E., Larsen, R.S., Ning, D., Boomsma, J.J., Zhang, G. 2020. The gene expression network regulating queen brain remodeling after insemination and its parallel use in ants with reproductive workers. Science Advances 6, eaaz5772 (doi:10.1126/sciadv.aaz5772).
- Nascimento FS, Souza DISA, Tannure-Nascimento IC and Dantas JO. 2012. Social facilitation and food partitioning in the queenless ant Dinoponera quadriceps (Hymenoptera: Formicidae). Journal of Natural History 46: 31–32.
- Nascimento FS, Tannure-Nascimento IC, Dantas JO and Turatti IC. 2012. Task-related variation of cuticular hydrocarbon profiles affect nestmate recognition in the giant ant Dinoponera quadriceps. Journal of Insect Behavior. doi: 10.1007/s10905-012-9353-5
- Patalano, S., A. Vlasova, C. Wyatt, P. Ewels, F. Camara, P. G. Ferreirab, C. L. Asher, T. P. Jurkowski, A. Segonds-Pichon, M. Bachman, I. Gonzalez-Navarrete, A. E. Minoche, F. Krueger, E. Lowy, M. Marcet-Houben, J. L. Rodriguez-Ales, F. S. Nascimento, S. Balasubramanian, T. Gabaldon, J. E. Tarver, S. Andrews, H. Himmelbauer, W. O. H. Hughes, R. Guigo, W. Reik, and S. Sumner. 2015. Molecular signatures of plastic phenotypes in two eusocial insect species with simple societies. Proceedings of the National Academy of Sciences of the United States of America. 112:13970-13975. doi:10.1073/pnas.1515937112
- Peeters, C., Monnin T. & Malosse, C. 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. Royal Society of London B 266: 1323-1327.
- Qiu, B., Larsen, R.S., Chang, N.-C., Wang, J., Boomsma, J.J., Zhang, G. 2018. Towards reconstructing the ancestral brain gene-network regulating caste differentiation in ants. Nature Ecology, Evolution 2, 1782–1791. (doi:10.1038/S41559-018-0689-X).
- Santos, I.S., Delabie, J.H.C., Silva, J.G., Costa, M.A., Barros, L.A.C., Pompolo, S.G. & Mariano, C.S.F. 2012. Karyotype differentiation among four Dinoponera (Formicidae: Ponerinae) species. Florida Entomologist 95(3), 737-742
- Schultner, E., Pulliainen, U. 2020. Brood recognition and discrimination in ants. Insectes Sociaux 67, 11–34 (doi:10.1007/s00040-019-00747-3).
- Silva, J. P., Valadares, L., Vieira, M. E. L., Teseo, S., Châline, N. 2021. Tandem running by foraging Pachycondyla striata workers in field conditions vary in response to food type, food distance, and environmental conditions. Current Zoology 67(5), 541–549 (doi:10.1093/cz/zoab050).
- Sousa PL, Quinet YP, Ponte EL, do Vale JF, Torres AFC, Pereira MG and Assreuy AMS. 2012. Venom’s antinociceptive property in the primitive ant Dinoponera quadriceps. Journal of Ethnoparmacology.
- Tannure-Nascimento IC, Nascimento FS, Dantes JO and Zucchi R. 2009. Decision rules for egg recognition are related to functional roles and chemical cues in the queenless ant Dinoponera quadriceps. Naturwissenschaften 96:857–861. doi: 10.1007/s00114-009-0535-8
- Touchard, A., Dejean, A., Escoubas, P., Orivel, J. 2015. Intraspecific variations in the venom peptidome of the ant Odontomachus haematodus (Formicidae: Ponerinae) from French Guiana. Journal of Hymenoptera Research 47, 87–101 (doi:10.3897/jhr.47.6804).
- Tozetto, L., Lattke, J.E. 2020. Revealing male genital morphology in the giant ant genus Dinoponera with geometric morphometrics. Arthropod Structure & Development 57, 100943 (doi:10.1016/j.asd.2020.100943)).
- Ulysséa, M.A., Brandão, C.R.F. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia 57, 217–224 (doi:10.1590/s0085-56262013005000002).
- Vasconcellos A, Santana GG and Souza AK. 2004. Nest spacing and architecture and swarming of males of Dinoponera quadriceps (Hymenoptera, Formicidae) in a remnant of the Atlantic Forest in Northeast Brazil. Brazilian Journal of Biology 64: 357–362. doi: 10.1590/S1519-69842004000200022
- Zocca, C., Curbani, F., Ferreira, R.B., Weichert, C., Sobrinho, T.G., Srbek-Araujo, A.C. 2021. A day in the life of the giant ant Dinoponera lucida Emery, 1901 (Hymenoptera, Formicidae): Records of activities and intraspecific interactions. Sociobiology 68, 6166 (doi:10.13102/sociobiology.v68i2.6166).
References based on Global Ant Biodiversity Informatics
- Kempf W. W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hymenoptera: Formicidae). Studia Entomologica 14: 369-394.
- Kempf W. W. 1975. Miscellaneous studies on neotropical ants. VI. (Hymenoptera, Formicidae). Studia Entomologica 18: 341-380.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Lenhart, P. A.; Dash, S. T.; and Mackay, W. P. 2013. A revision of the giant Amazonian ants of the genus Dinoponera (Hymenoptera, Formicidae). Journal of Hymenoptera Research 31: 119-164
- Monnin, T. and C. Peeters. 1997. Cannibalism of subordinates eggs in the monogynous queenless ant Dinoponera quadriceps. Naturwissenschaften 84, 499502
- Nunes F. A., G. B. Martins Segundo, Y. B. Vasconcelos, R. Azevedo, and Y. Quinet. 2011. Ground-foraging ants (Hymenoptera: Formicidae) and rainfall effect on pitfall trapping in a deciduous thorn woodland (Caatinga), Northeastern Brazil. Rev. Biol. Trop. 59 (4): 1637-1650.
- Santos I. S., J. H. C. Delabie, J. G. Silva, M. A. Costa, L. A. C. Barros, S. G. Pompolo, and C. S. F. Mariano. 2012. Karyotype differentiation among four Dinoponera (Formicidae: Ponerinae) species. Florida Entomologist 95(3): 737-742.
- Ulyssea M. A., and C. R. F. Brandao. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia 57(2): 217224.
- Ulysséa M. A., C. R. F. Brandão. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia 57(2): 217-224.