Paratrechina longicornis
| Paratrechina longicornis | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Lasiini |
| Genus: | Paratrechina |
| Species: | P. longicornis |
| Binomial name | |
| Paratrechina longicornis (Latreille, 1802) | |
| Synonyms | |
| |
| Common Name | |
|---|---|
| Crazy Ant | |
| Language: | English |
| Higenaga-ameiro-ari | |
| Language: | Japanese |
This ant has been transported to almost all the populated subtropical and tropical areas in the world. It is usually in disturbed areas but can invade undisturbed areas as well. It is a general scavenger and also tends honeydew-producing Homoptera. Nests are in accumulations of dry litter or mulch or under objects on the ground.
| At a Glance | • Highly invasive • Polygynous • Supercolonies • Parthenogenetic |
Photo Gallery
Identification
Keys including this Species
- Key to Paratrechina Species
- Key to Micronesian Ants
- Key to Paratrechina of the southwestern Australian Botanical Province
- Key to workers of the Socotra Archipelago, Yemen
- Key to Hispaniola Genera of Formicinae
Distribution
A common tramp ant that has been spread to most of the world's subtropical and tropical areas with substantial human populations.
Latitudinal Distribution Pattern
Latitudinal Range: 17.201° to -33.93333°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Afrotropical Region: Benin, Cameroun, Chad, Comoros, Democratic Republic of Congo, Eritrea, Gambia, Guinea, Kenya, Malawi, Mali, Mauritania, Mozambique, Nigeria, Saint Helena, Senegal (type locality), Socotra Archipelago, Uganda, United Arab Emirates, United Republic of Tanzania, Yemen.
Australasian Region: Australia, New Caledonia, Norfolk Island.
Indo-Australian Region: Borneo, Brunei Darussalam, Cook Islands, Fiji, Guam, Hawaii, Indonesia, Kiribati, Krakatau Islands, Malaysia, Marshall Islands, Micronesia (Federated States of), New Guinea, Niue, Northern Mariana Islands, Palau, Philippines, Samoa, Singapore, Solomon Islands, Timor-Leste, Tokelau, Tonga, Tuvalu, Vanuatu.
Malagasy Region: Madagascar, Mauritius, Mayotte, Réunion, Seychelles.
Nearctic Region: United States.
Neotropical Region: Anguilla, Aruba, Bahamas, Barbados, Belize, Bermuda, Brazil, Cayman Islands, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, French Guiana, Galapagos Islands, Greater Antilles, Grenada, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Lesser Antilles, Mexico, Netherlands Antilles, Nicaragua, Panama, Paraguay, Peru, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Trinidad and Tobago, Turks and Caicos Islands, Venezuela.
Oriental Region: Bangladesh, Cambodia, India, Laos, Maldives, Nepal, Nicobar Island, Pakistan, Sri Lanka, Thailand, Vietnam.
Palaearctic Region: Bahrain, Balearic Islands, Belgium, Canary Islands, China, Cyprus, Estonia, France, Greece, Iberian Peninsula, Iran, Israel, Italy, Japan, Libya, Malta, Oman, Spain, Türkiye, United Kingdom of Great Britain and Northern Ireland.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
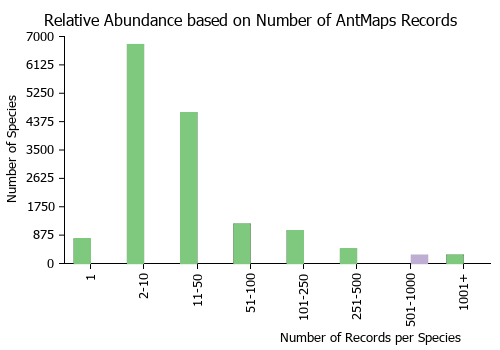
|
Biology
San Cristóbal, República Dominicana. Video by Judá Isaí Martínez Uribe.
Sharaf et al. (2017) report on this species in Yemen: The known habitats of this species are diverse, no doubt enabling its wide distribution. We observed Paratrechina longicornis nesting in moist soil under a rock adjacent to a date palm tree. Another nest was collected from dry soil under shrub Frangula alnus Mill. (Rhamnaceae). Many workers were found in leaf litter under a date palm tree where the soil was moist and rich in accumulated sheep and goat faeces. Several workers were foraging in leaf litter on dry soil under an Eragrostis tef (Zucc.) Trotter (Poaceae) tree, where the soil was dry. A nest was observed under a rock in moist, compacted, clay soil. Hundreds of workers were foraging in moist leaf litter and on twigs of a small shrub. Several workers were nesting under a stone in humid soil and next to banana plantations. A nest was found under a rock next to a dragon blood tree, Dracaena cinnabari Balf.f. (Asparagaceae). This species has been reported as a pest in greenhouses in both temperate and tropical regions (Nylander 1856; Motschoulsky 1863).
Bertelsmeier et al. (2015) examined elements of interspecific aggression between this species and several other highly invasive ants. In laboratory assays Paratrechina longicornis was adept at avoiding aggressive interactions. When confronted by workers of other invasive ant species P. longicornis either acted indifferently or moved away.
Florida (USA) - Introduced into Florida and found as far north and west as Leon County, but much commoner in south Florida. Pest status: a minor nuisance in outdoor eating areas, and frequently enters buildings where there is easy access to the outside. First published Florida record: Smith 1930. (Deyrup, Davis & Cover, 2000.)
In Benin, Taylor et al. (2018) found this species in mango (Mangifera indica) orchards cohabiting with Oecophylla longinoda and running on the trunk of Terminalia catappa.
Foraging
McCreery et al. (2019) tested movement initiation and obstacle navigation in group foraging. Baits of various sizes were used, with testing conducted in the field in Tempe, Arizona. They found P. longicornis excel at cooperative transport despite wide load variation. The effects of load properties vary across transport challenges, and groups struggling to move large loads may find obstacle navigation easier once a load has begun to be moved.
Nesting Habits
Jaffe (1993) reported that at low tide on the beaches of Mumbai, India, hundreds of meters of new coast are exposed, filled with marine debris and human detritus. In this area, some 100 m from the high tide line, he found colonies of Paratrechina longicornis nesting in the sand and foraging on the debris around their nest up to 25 m away. Nest density was very high (estimated in over 1 nest/ m2), although as colonies are known to be polydomic and all nests likely formed a single colony. These ants have to hide in their nests at high tide, which probably are protected from flooding thanks to trapped air in their galleries.
Chemical Ecology
LeBrun et al. (2015) found a behaviour, first noted and resulting from interactions between Solenopsis invicta and Nylanderia fulva, that detoxifies fire ant venom is expressed widely across ants in the subfamily Formicinae. This behavior was also studied and shown in experiments with P. longicornis. See the biology section of the N. fulva page for a description of acidopore grooming and the use of formic acid for detoxification of a specific class of venoms that are produced by ants that may interact with formicines in the context of predation and food competition.
Regional Notes
Borowiec and Salata (2022), Greece - Invasive thermophilous species, noted from tourist resorts. Inhabits ruderal areas, grasslands and parks. Nests in cavities in plants and trees, rotten wood, and in soil. Workers are omnivorous, feeding on live and dead insects, seeds, honeydew, fruits, plant exudates, and many household foods. All records are from low altitude from sea level to 155 m.
Flight Period
| X | X | ||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
 Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a mutualist for the aphid Aphis coreopsidis (a trophobiont) (Favret et al., 2010; Saddiqui et al., 2019).
- This species is a mutualist for the aphid Aphis craccivora (a trophobiont) (Shiran et al., 2013; Rakhshan and Ahmad, 2015; Saddiqui et al., 2019).
- This species is a mutualist for the aphid Aphis gossypii (a trophobiont) (Idechiil et al., 2007; Lokeshwari et al., 2015; Saddiqui et al., 2019).
- This species is a host for the cricket Myrmecophilus americanus (a myrmecophile).
- This species is a prey for the tiger beetle Cicindela duponti (a predator) in Western Ghats, India (Sinu et al., 2006).
- This species is a associate (details unknown) for the fungus Absidia corymbifera (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Acremonium sp. (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Aspergillus candidus (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Aspergillus ochraceus (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Aspergillus versicolor (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Cladosporium sp. (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Cunninghamella equinalata (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Cunninghamella polymorpha (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Geotrichum sp. (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Penicillium sp. (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Purpureocillium lilacinum (a associate (details unknown)) (Quevillon, 2018).
- This species is a host for the fungus Aspergillus flavus (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission within nest).
- This species is a host for the fungus Metarhizium anisopliae (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission within nest).
Life History Traits
- Queen number: polygynous (Holldobler & Wilson, 1977; Frumhoff & Ward, 1992)
Castes
Worker
                
| |
| . | |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0101797. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by MHNG, Geneva, Switzerland. |
   
| |
| Queen (alate/dealate). Specimen code casent0132425. Photographer Erin Prado, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
    
| |
| Queen (alate/dealate). Specimen code casent0137340. Photographer Erin Prado, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
    
| |
| Queen (alate/dealate). Specimen code casent0173230. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CDRS, Galapagos, Ecuador. |
Male
Images from AntWeb
    
| |
| Male (alate). Specimen code casent0173231. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CDRS, Galapagos, Ecuador. |
     
| |
| Male (alate). Specimen code casent0137341. Photographer Erin Prado, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Additional images can be found here.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- longicornis. Formica longicornis Latreille, 1802c: 113 (w.) SENEGAL. Jerdon, 1851: 124 (q.); André, 1881b: 60 (m.); Hung, Imai & Kubota, 1972: 1024 (k.); Wheeler, G.C. & Wheeler, J. 1986d: 336 (l.); Fox, et al. 2007: 3 (l.). Combination in Prenolepis: Roger, 1863b: 10; in Pr. (Nylanderia): Emery, 1910a: 129; in Paratrechina: Wheeler, W.M. 1921e: 112. Senior synonym of currens: Emery, 1892b: 166; of gracilescens: Roger, 1863b: 10; of vagans: Dalla Torre, 1893: 179. Senior synonym of hagemanni: LaPolla, Brady & Shattuck, 2010a: 128. See also: Mayr, 1865: 50; Forel, 1891b: 81; Forel, 1894c: 406; Emery, 1910a: 129; Trager, 1984b: 153.
- vagans. Formica vagans Jerdon, 1851: 124 (w.q.) INDIA. [Unresolved junior primary homonym of Formica vagans Olivier, 1792: 501.] Junior synonym of longicornis: Dalla Torre, 1893: 179; Forel, 1894c: 408.
- gracilescens. Formica gracilescens Nylander, 1856a: xxviii (w.) FRANCE. [Also described as new by Nylander, 1856b: 73.] Junior synonym of longicornis: Roger, 1863b: 10.
- currens. Paratrechina currens Motschoulsky, 1863: 14 (w.) SRI LANKA. Junior synonym of longicornis: Emery, 1892b: 166. Neotype designated: LaPolla, Brady & Shattuck, 2010b: 1.
- hagemanni. Prenolepis longicornis var. hagemanni Forel, 1901h: 65 (w.) DEMOCRATIC REPUBLIC OF CONGO. Combination in Paratrechina: Emery, 1925b: 217. Junior synonym of longicornis: Wheeler, W.M. 1922a: 942. Revived from synonymy: Emery, 1925b: 217. Junior synonym of longicornis: LaPolla, Brady & Shattuck, 2010a: 128.
Type Material
- Formica longicornis: Syntype, worker(s), Senegal.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
LaPolla et al. (2013) - (n=4): TL: 2.1- 2.5; HW: 0.46-56; HL: 0.49-0.7; EL: 0.17-0.23; SL: 0.98-1.16; PW: 0.34-0.43; WL: 0.82-0.98; PrFL: 0.6-0.9; GL: 0.83-0.9. Indices: CI: 73-94; REL2: 38-42 ; SI: 182-226.
Overall coloration pale to very dark brown, often with a distinct blueish iridescent sheen, especially on the mesosoma and gaster. Mandibles, antennae and legs (especially the trochanters of all legs, which are a strongly contrasting very pale yellow-brown) much lighter in color; cuticle smooth and moderately shining with faint shagreenate sculpture, which is most obvious on head and gaster. Head narrow, distinctly longer than broad, with abundant pale (yellow-brown to almost white), erect macrosetae; anterior clypeal margin with a shallow medial indentation; scapes with a dense layer of very fine pubescence but lacking erect macrosetae; eyes large and convex, extending beyond head lateral margin in full frontal view; posterior head margin with rounded posterolateral corners; three distinct ocelli present. Mesosoma with scattered pale erect macrosetae (PSC = 3; MSC = 3-4); in profile pronotum and mesonotum almost flat dorsally, with a broadly angled junction; metanotal area relatively indistinct, medially about 1/5 the length of the mesonotum but longer laterally than medially; dorsum of propodeum almost flat to very shallowly domed, rounding evenly into the short declivitious face; anterolateral portion of dorsal face with some scattered pubescence. Gaster with abundant erect pale macrosetae.
Borowiec and Salata (2022) - Large, HL: 0.627-0.714 (mean 0.676); HW: 0,444-0.524 (mean 0.483); SL: 1.063-1.222 (mean 1.138); EL: 0.178-0.222 (mean 0.197); ML: 0.93-1.06; MW: 0.34-0.40. Color. Body uniformly yellowish brown to dark brown, mandibles, antennae, and legs yellow, only in the darkest forms legs slightly infuscate. Head. Approximately 1.4 times longer than wide, sides almost parallel, occipital margin softly convex. Clypeus with diffused microreticulation, appears almost smooth and shiny, transverse, its anterior margin almost straight with shallow median emargination, posterior margin concave in the middle, clypeal surface with sparse and short appressed white pubescence, a row of long yellow setae at anterior margin and mixed short and long erected setae, the longest with length 0.152. Whole head with diffused microreticulation but appears shiny, with rudiments of sparse and short white appressed hairs and numerous very long, yellowish white, semierect to erected setae, the shortest on gena, the longest in interocular and occipital area, the longest with length 0.183. Ventral sides of head with several, long, white erected setae grouping mostly on sides of gular area. Scape very long, 2.31-2.41 times longer than width of head, only slightly widened from base to apex, its surface microreticulate but shiny with very short and sparse appressed pubescence, erected setae absent or at most in apical third of frontal edge with single, short erect seta. Funicular segments elongate, thin, the second funicular segment approximately 2.2 times as long as wide, slightly shorter than first and distinctly shorter than third segment, the rest of funicular segments very long, clearly longer than broad. Eyes big, distinctly longer than wide, 0.29 length of head. Mandibles elongate, without longitudinal sculpture. Mesosoma. Strongly elongate, softly constricted in the middle, 2.4-2.8 times as long as wide, surface without appressed pubescence, with diffused microreticulation, appears slightly opaque. In lateral view promesonotum almost linear, metanotal groove very shallow, propodeum elongate, softly convex. The whole surface of promesonotum with numerous, very long, white or yellowish white erected setae, as long as the longest setae on head, propodeum without setae. Waist and gaster. Petiole small, in form of thick scale with rounded apex, without setae in anterolateral corners. Gaster shorter than mesosoma, surface of tergites distinctly microreticulated, without appressed pubescence, appears slightly opaque, each tergite with numerous very long, white to yellowish erected setae, the longest with length 0.190. Legs thin and elongate, femora distinctly longer than gaster. Legs. Elongate, surface of femora and tibiae with several semierect white setae.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- n = 8, 2n = 16, karyotype = 14M+2A (India) (Imai et al., 1984).
- n = 16 (Indonesia) (Imai et al., 1985).
- 2n = 16 (Taiwan) (Hung et al., 1972).
References
- Abdar, M.R. 2020. Seasonal abundance and commonly occurring household ants species in Sangli District Maharashtra. Research Journal of Agricultural Sciences 11(6): 1413-1415.
- Ahmad, Z., Ghramh, H.A., Ali Khan, K., Khan, F., Shujauddin. 2020. Impact of Two Attending Ants, Crematogaster subnuda and Camponotus compressus (Hymenoptera: Formicidae), on the Parasitism of Sugarcane Aphid Melanaphis sacchari (Zehnt.) Pakistan Journal of Zoology (doi:10.17582/journal.pjz20200309190337).
- Akhila, A., Keshamma, E. 2022. A review on diversity of ant species in Karnataka State. International Journal of Advanced Scientific Research and Management 7(6): 15-20 (doi:10.36282/IJASRM/7.6.2022.1865).
- Alatorre-Bracamontes, C.E., Vásquez-Bolaños, M. 2010. Lista comentada de las hormigas (Hymenoptera: Formicidae) del norte de México. Dugesiana 17(1): 9-36.
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- André, E. 1881b. [Untitled. Introduced by: "M. Ernest André, de Gray, adresse les descriptions de trois nouvelles espèces de Fourmis".]. Bull. Bimens. Soc. Entomol. Fr. 1881: 60-62 (page 60, male described)
- Ashigar, M.A., Ab Majid, A.H. 2020. Diversity, abundance, and foraging behavior of ants (Hymenoptera: Formicidae) scavenging on American Cockroach in various habitats of Nasarawa State, Nigeria. Pertanika Journal of Tropical Agricultural Science 43: 503-521 (doi:10.47836/pjtas.43.4.07).
- Baena, M.L., Escobar, F., Valenzuela, J.E. 2019. Diversity snapshot of green–gray space ants in two Mexican cities. International Journal of Tropical Insect Science 40, 239–250 (doi:10.1007/s42690-019-00073-y).
- Baidya, P., Bagchi, S. 2021. Influence of human land use and invasive species on beta diversity of tropical ant assemblages. Insect Conservation and Diversity, icad.12536 (doi:10.1111/icad.12536).
- Baltazar, C.R. 1966. A catalogue of Philippine Hymenoptera (with a bibliography, 1758-1963). Pacific Insects Monographs 8: 1-488. (page 267, listed)
- Barros, L.A.C., Rabeling, C., Teixeira, G.A., dos Santos Ferreira Mariano, C., Delabie, J. H. C., de Aguiar, H. J. A. C. 2022. Decay of homologous chromosome pairs and discovery of males in the thelytokous fungus-growing ant Mycocepurus smithii. Scientific Reports 12, 4860 (doi:10.1038/s41598-022-08537-x).
- Bertelsmeier, C., A. Avril, O. Blight, A. Confais, L. Diez, H. Jourdan, J. Orivel, N. St Germes, and F. Courchamp. 2015. Different behavioural strategies among seven highly invasive ant species. Biological Invasions. 17:2491-2503. doi:10.1007/s10530-015-0892-5
- Bharti, H., Sharma, Y.P., Kaur, A. 2009. Seasonal patterns of ants (Hymenoptera: Formicidae) in Punjab Shivalik. Halteres 1: 36-47.
- Blard, F., Dorow, W.-H.-O., Delabie, J. H. C. 2003. Les Fourmis de l’île de la Réunion (Hymenoptera, Formicidae). Bulletin de La Société Entomologique de France, 108(2), 127–137 (doi:10.3406/bsef.2003.16939).
- Blum, M. S.; Wilson, E. O. 1964. The anatomical source of trail substances in formicine ants. Psyche (Cambridge) 71:28-31. [1964-05-05]
- Borowiec, L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Borowiec, L., Salata, S. 2018. Notes on ants (Hymenoptera: Formicidae) from Gambia (Western Africa). Annals of the Upper Silesian Museum in Bytom, Entomology 26 (online 010), 1-13 (doi:10.5281/ZENODO.1243767).
- Borowiec, L., Salata, S. 2022. A monographic review of ants of Greece (Hymenoptera: Formicidae). Vol. 1. Introduction and review of all subfamilies except the subfamily Myrmicinae. Part 1: text. Natural History Monographs of the Upper Silesian Museum 1: 1-297.
- Borowiec, L., Wieczorek, K., Salata, S. 2021. Review of ants (Hymenoptera: Formicidae) of the Dodecanese Archipelago, Greece. Annals of the Upper Silesian Museum in Bytom Entomology 30: 1-33 (doi:10.5281/ZENODO.5571270).
- Brassard, F., Leong, C.-M., Chan, H.-H., Guénard, B. 2021. High diversity in urban areas: How comprehensive sampling reveals high ant species richness within one of the most urbanized regions of the world. Diversity 13, 358 (doi:10.3390/d13080358).
- Brühl, C.A., Eltz, T. 2009. Fuelling the biodiversity crisis: species loss of ground-dwelling forest ants in oil palm plantations in Sabah, Malaysia (Borneo). Biodiversity and Conservation 19, 519–529 (doi:10.1007/s10531-009-9596-4).
- Bulter, I. 2020. Hybridization in ants. Ph.D. thesis, Rockefeller University.
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Catarineu, C., Barberá, G.G., Reyes-López, J.L. 2018. Zoogeography of the ants (Hymenoptera: Formicidae) of southeastern Iberian Peninsula. Sociobiology 65, 383-396 (doi:10.13102/sociobiology.v65i3.2822).
- Chanda, A. 2017. A study on ants (Hymenoptera: Formicidae) of Medinipur, West Bengal, India. International Journal of Entomological Research 2(5): 1-4.
- Collingwood, C. A., Pohl, H., Guesten, R., Wranik, W. and van Harten, A. 2004. The ants (Insecta: Hymenoptera: Formicidae) of the Socotra Archipelago. Fauna of Arabia. 20:473-495.
- Dalla Torre, K. W. von. 1893. Catalogus Hymenopterorum hucusque descriptorum systematicus et synonymicus. Vol. 7. Formicidae (Heterogyna). Leipzig: W. Engelmann, 289 pp. (page 179, Senior synonym of vagans)
- Dekoninck, W., Ignace, D., Vankerkhoven, F., Wegnez, P. 2012. Verspreidingsatlas van de mieren van België. Bulletin de la Société royale belge d’Entomologie 148: 95-186.
- Dekoninck, W., Wauters, N., Delsinne, T. 2019. Capitulo 35. Hormigas invasoras en Colombia. Hormigas de Colombia.
- Demetriou, J., Georgiadis, C., Ralli, V., Salata, S., Borowiec, L. 2024. Setting the record straight: a re-examination of ants (Hymenoptera: Formicidae) from Cyprus deposited at the Museum of Zoology of Athens. Zootaxa 55231, 49–69 (doi:10.11646/zootaxa.5523.1.3).
- Deyrup, M., Davis, L. & Cover, S. 2000. Exotic ants in Florida. Transactions of the American Entomological Society 126, 293-325.
- Deyrup, M.A., Carlin, N., Trager, J., Umphrey, G. 1988. A review of the ants of the Florida Keys. Florida Entomologist 71: 163-176.
- Dias, R.K.S., Kosgamage, K.R.K.A. 2013. Occurrence and species diversity of ground-dwelling worker ants (Family: Formicidae) in selected lands in the dry zone of Sri Lanka. Journal of Science of the University of Kelaniya Sri Lanka 7: 55-72 (doi:10.4038/josuk.v7i0.6233).
- Dias, R.K.S., Rajapaksa, R.P.K.C. 2017. Geographic records of subfamilies, genera and species of ants (Hymenoptera: Formicidae) in the four climatic zones of Sri Lanka: A review. Journal of Science of the University of Kelaniya Sri Lanka 11, 23-45. (doi:10.4038/josuk.v11i2.7999).
- do Nascimento, L.E., Amaral, R.R., Ferreira, R.M.dos A., Trindade, D.V.S., do Nascimento, R.E., da Costa, T.S., Souto, R.N.P. 2020. Ants (Hymenoptera: Formicidae) as potential mechanical vectors of pathogenic bacteria in a public hospital in the Eastern Amazon, Brazil. Journal of Medical Entomology 57: 1619–1626. (doi:10.1093/JME/TJAA062).
- Egbon, I.N., Osabuohien, I.P. 2022. First checklist, species richness and diversity of leaf-litter dwelling ants (Hymenoptera: Formicidae) in ancient Benin moat, Nigeria. Animal Research International 19(3): 4634–4642.
- Emery, C. 1892c [1891]. Note sinonimiche sulle formiche. Bull. Soc. Entomol. Ital. 23: 159-167 (page 166, Senior synonym of currens)
- Emery, C. 1910a. Beiträge zur Monographie der Formiciden des paläarktischen Faunengebietes. (Hym.) Teil X. Dtsch. Entomol. Z. 1910: 127-132 (page 129, Combination in Pr. (Nylanderia))
- Eyer, P.-A., Blumenfeld, A.J., Vargo, E.L. 2019. Sexually antagonistic selection promotes genetic divergence between males and females in an ant. Proceedings of the National Academy of Sciences 116, 24157–24163 (doi:10.1073/PNAS.1906568116).
- Fontenla, J.L., Brito, Y.M. 2011. Hormigas invasoras y vagabundas de Cuba. Fitosanidad 15(4), 253-259.
- Forel, A. 1891c. Les Formicides. [part]. In: Grandidier, A. Histoire physique, naturelle, et politique de Madagascar. Volume XX. Histoire naturelle des Hyménoptères. Deuxième partie (28e fascicule). Paris: Hachette et Cie, v + 237 pp. (page 81, see also)
- Forel, A. 1894c. Les Formicides de l'Empire des Indes et de Ceylan. Part IV. J. Bombay Nat. Hist. Soc. 8: 396-420 (page 406, see also)
- Fournier, D., Tindo, M., Kenne, M., Mbenoun Masse, P.S., Van Bossche, V., De Coninck, E., Aron, S. 2012. Genetic structure, nestmate recognition and behaviour of two cryptic species of the invasive Big-Headed Ant Pheidole megacephala. PLoS ONE 7(2): e31480 (doi:10.1371/journal.pone.0031480).
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Guillem, R., Bensusan, K. 2022. Thee new exotic species of ants (Hymenoptera, Formicidae) for Madeira, with comments on its myrmecofauna. Journal of Hymenoptera Research 91: 321–333 (doi:10.3897/jhr.91.81624).
- Hashimoto, Y. 1990. Unique features of sensilla on the antennae of Formicidae (Hymenoptera). Applied Entomology and Zoology 25: 491-501.
- Hasin, S., Tasen, W. 2020. Ant community composition in urban areas of Bangkok, Thailand. Agriculture and Natural Resources 54: 507-514 (doi:10.34044/j.anres.2020.54.5.07).
- Herrera, H.W., Baert, L., Dekoninck, W., Causton, C.E., Sevilla, C.R., Pozo, P., Hendrickx, F. 2020. Distribution and habitat preferences of Galápagos ants (Hymenoptera: Formicidae). Belgian Journal of Entomology, 93: 1–60.
- Heterick, B.E. 2021. A guide to the ants of Western Australia. Part I: Systematics. Records of the Western Australian Museum, Supplement 86, 1-245 (doi:10.18195/issn.0313-122x.86.2021.001-245).
- Heterick, B.E. 2022. A guide to the ants of Western Australia. Part II: Distribution and biology. Records of the Western Australian Museum, supplement 86: 247-510 (doi:10.18195/issn.0313-122x.86.2022.247-510).
- Heterick, B.E., Kitching, R.L. 2022. The ants (Hymenoptera: Formicidae) of a one-hectare plot of lowland dipterocarp forest. Entomologist’s Monthly Magazine 158(4), 261–272 (doi:10.31184/m00138908.1584.4153).
- Hoey-Chamberlain, R., Rust, M.K. 2014. Food and bait preferences of Liometopum occidentale (Hymenoptera: Formicidae). Journal of Entomological Science 49(1): 30-43.
- Hoey-Chamberlain, R.V. 2012. Food preference, survivorship, and intraspecific interactions of Velvety Tree Ants. M.S. thesis, University of California, Riverside.
- Hoffmann, B., Eldridge, J., Marston, C. 2023. The first eradication of an exotic ant species from the entirety of Australia: Pheidole fervens. Management of Biological Invasions, 14(4), 619–624 (doi:10.3391/mbi.2023.14.4.03).
- Hung, A. C. F.; Imai, H. T.; Kubota, M. 1972. The chromosomes of nine ant species (Hymenoptera: Formicidae) from Taiwan, Republic of China. Ann. Entomol. Soc. Am. 6 65: 1023-1025 (page 1024, karyotype described)
- Imai, H.T., Kihara, A., Kondoh, M., Kubota, M., Kuribayashi, S., Ogata, K., Onoyama, K., Taylor, R.W., Terayama, M., Yoshimura, M., Ugawa, Y. 2003. Ants of Japan. 224 pp, Gakken, Japan.
- Ipser, R.M., Brinkman, M.A., Gardner, W.A., Peeler, H.B. 2004. A survey of ground-dwelling ants (Hymenoptera: Formicidae) in Georgia. Florida Entomologist 87: 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87 (doi:10.3897@jhr.70.35207).
- Jaffe, K. 1993. Surfing ants. Florida Entomologist 76: 182-183.
- Jerdon, T. C. 1851. A catalogue of the species of ants found in Southern India. Madras J. Lit. Sci. 17: 103-127 (page 124, queen described)
- Karaman, C., Kıran, K. 2018. New tramp ant species for Turkey: Tetramorium lanuginosum Mayr (Hymenoptera: Formicidae). Trakya University Journal of Natural Sciences 19(1), e1-e4 (doi:10.23902/trkjnat.340008).
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Kiran, K., Karaman, C. 2020. Additions to the ant fauna of Turkey (Hymenoptera, Formicidae). Zoosystema 42(18), 285-329 (doi:10.5252/zoosystema2020v42a18).
- Kreider, J.J., Chen, T.W., Hartke, T.R., Buchori, D., Hidayat, P., Nazarreta, R., Scheu, S., Drescher, J. 2021. Rainforest conversion to monocultures favors generalist ants with large colonies. Ecosphere 12 (doi:10.1002/ecs2.3717).
- Landero-Torres, I., Garcia-Martinez, M.A., Galindo-Tovar, M.E., Leyva-Ovalle, O.R., Lee-Espinosa, H.E., Murguia-Gonzalez, J., Negrin-Ruiz, J. 2014. Alpha diversity of the myrmecofauna of the Natural Protected Area Metlac from Fortin, Veracruz, Mexico. Southwestern Entomologist 39: 541-553.
- LaPolla, J.S., Hawkes, P.G., Fisher, J.N. 2013. Taxonomic review of the ant genus Paratrechina, with a description of a new species from Africa. Journal of Hymenoptera Research 35: 71–82 (doi: 10.3897/JHR.35.5628)
- Latreille, P.A. 1802. Histoire naturelle des fourmis, et recueil de mémoires et d'observations sur les abeilles, les araignées, les faucheurs, et autres insectes. Paris: Impr. Crapelet (chez T. Barrois), xvi + 445 pp.
- Latumahina, F., Borovanska, M., Musyafa, Sumardi, Susetya Putra, N., Janda, M. 2015. Ants of Ambon Island – diversity survey and checklist. ZooKeys 472, 43–57 (doi:10.3897/zookeys.472.8441).
- LeBrun, E. G., P. J. Diebold, M. R. Orr, and L. E. Gilbert. 2015. Widespread Chemical Detoxification of Alkaloid Venom by Formicine Ants. Journal of Chemical Ecology. 41:884-895. doi:10.1007/s10886-015-0625-3
- Lee, C.-C., Weng, Y.-M., Lai, L.-C., Suarez, A.V., Wu, W.-J., Lin, C.-C., Yang, C.-C.S. 2020. Analysis of recent interception records reveals frequent transport of arboreal ants and potential predictors for ant invasion in Taiwan. Insects 11, 356 (doi:10.3390/INSECTS11060356).
- Liu, C., Fischer, G., Hita Garcia, F., Yamane, S., Liu, Q., Peng, Y.Q., Economo, E.P., Guénard, B., Pierce, N.E. 2020. Ants of the Hengduan Mountains: a new altitudinal survey and updated checklist for Yunnan Province highlight an understudied insect biodiversity hotspot. ZooKeys 978, 1–171 (doi:10.3897/zookeys.978.55767).
- Lubertazzi, D. 2019. The ants of Hispaniola. Bulletin of the Museum of Comparative Zoology, 162(2), 59-210 (doi:10.3099/mcz-43.1).
- Lutinski, J., de Filtro, M., Baucke, L., Dorneles, F., Lutinski, C., Guarda, C. 2021. Ant assemblages (Hymenoptera: Formicidae) from areas under the direct influence of two small hydropower plants in Brazil. Brazilian Journal of Environmental Sciences (Online), 1-9 (doi:10.5327/Z217694781030).
- MacGown, J.A., Booher, D., Richter, H., Wetterer, J.K., Hill, J.G. 2021. An updated list of ants of Alabama (Hymenoptera: Formicidae) with new state records. Transactions of the American Entomological Society 147: 961-981 (doi:10.3157/061.147.0409).
- Majer, J.D., Castalanelli, M.A., Ledger, J.L., Gunawardene, N.R., Heterick, B.E. 2018. Sequencing the ant fauna of a small island: Can metagenomic analysis enable faster identification for routine ant surveys? Sociobiology 65, 422-432 (doi:10.13102/sociobiology.v65i3.2885).
- Mayr, G. 1865. Formicidae. In: Reise der Österreichischen Fregatte "Novara" um die Erde in den Jahren 1857, 1858, 1859. Zoologischer Theil. Bd. II. Abt. 1. Wien: K. Gerold's Sohn, 119 pp. (page 50, see also)
- Melo, T.S., Koch, E.B.A., Andrade, A.R.S., Travassos, M.L.O., Peres, M.C.L., Delabie, J.H.C. 2021. Ants (Hymenoptera: Formicidae) in different green areas in the metropolitan region of Salvador, Bahia state, Brazil. Brazilian Journal of Biology 82, e236269 (doi:10.1590/1519-6984.236269).
- Meurgey, F. 2020. Challenging the Wallacean shortfall: A total assessment of insect diversity on Guadeloupe (French West Indies), a checklist and bibliography. Insecta Mundi 786: 1–183.
- Mohseni, M.R., Pashaei Rad, S. 2021. The effect of edaphic factors on the distribution and abundance of ants (Hymenoptera: Formicidae) in Iran. Biodiversity Data Journal 9, e54843 (doi:10.3897/bdj.9.e54843).
- Molfini, M., Zapparoli, M., Genovesi, P., Carnevali, L., Audisio, P., Di Giulio, A., Bologna, M.A. 2020. A preliminary prioritized list of Italian alien terrestrial invertebrate species. Biological Invasions 22(8), 2385–2399 (doi:10.1007/s10530-020-02274-w).
- Moura, M.N., Cardoso, D.C., Cristiano, M.P. 2020. The tight genome size of ants: diversity and evolution under ancestral state reconstruction and base composition. Zoological Journal of the Linnean Society, zlaa135 (doi:10.1093/zoolinnean/zlaa135).
- Mukherjee, A., Roy, U.S. 2021. Role of physicochemical parameters on diversity and abundance of ground–dwelling, diurnal ant species of Durgapur Government College Campus, West Bengal, India. Munis Entomology and Zoology Journal 16(1): 366-380.
- Narváez-Vásquez, A., Gaviria, J., Vergara-Navarro, E.V., Rivera-Pedroza, L., Löhr, B. 2021. Ant (Hymenoptera: Formicidae) species diversity in secondary forest and three agricultural land uses of the Colombian Pacific Coast. Revista Chilena de Entomologia 47, 441–458 (doi:10.35249/rche.47.3.21.01).
- Nazarreta, R., Hartke, T.R., Hidayat, P., Scheu, S., Buchori, D., Drescher, J. 2020. Rainforest conversion to smallholder plantations of rubber or oil palm leads to species loss and community shifts in canopy ants (Hymenoptera: Formicidae). Myrmecological news 30, 175-186 (doi:10.25849/MYRMECOL.NEWS_030:175).
- Oussalah, N., Marniche, F., Espadaler, X., Biche, M. 2019. Exotic ants from the Maghreb (Hymenoptera, Formicidae) with first report of the hairy alien ant Nylanderia jaegerskioeldi (Mayr) in Algeria. Arxius de Miscel·lània Zoològica, 45–58 (doi:10.32800/amz.2019.17.0045).
- Pearcy, M., Goodisman, M.A.D., Keller, L. 2011. Sib mating without inbreeding in the longhorn crazy ant. Proceedings of the Royal Society London B 278: 2677–2681 (doi:10.1098/rspb.2010.2562).
- Rafael, J.A., Limeira-de-Oliveira, F., Hutchings, R.W., Miranda, G.F.G., Silva Neto, A.M.da, Somavilla, A., Camargo, A., Asenjo, A., Pinto, Â.P., Bello, A.de M., Dalmorra, C., Mello-Patiu, C.A.de, Carvalho, C.J.B.de, Takiya, D.M., Parizotto, D.R., Marques, D.W.A., Cavalheiro, D.de O., Mendes, D.M.de M., Zeppelini, D., Carneiro, E., Lima, É.F.B., Lima, E.C.A.de, Godoi, F.S.P.de, Pessoa, F.A.C., Vaz-de-Mello, F.Z., Sosa-Duque, F.J., Flores, H.F., Fernandes, I.O., Silva-Júnior, J.O., Gomes, L.R.P., Monné, M.L., Castro, M.C.M.de, Silva, M.P.G.da, Couri, M.S., Gottschalk, M.S., Soares, M.M.M., Monné, M.A., Rafael, M.S., Casagrande, M.M., Mielke, O.H.H., Grossi, P.C., Pinto, P.J.C., Bartholomay, P.R., Sobral, R., Heleodoro, R.A., Machado, R.J.P., Corrêa, R.C., Hutchings, R.S.G., Ale-Rocha, R., Santos, S.D.dos, Lima, S.P.de, Mahlmann, T., Silva, V.C., Fernandes, D.R.R. 2020. Insect (Hexapoda) diversity in the oceanic archipelago of Fernando de Noronha, Brazil: updated taxonomic checklist and new records. Revista Brasileira de Entomologia 64, e20200052 (doi:10.1590/1806-9665-rbent-2020-0052).
- Rafiqi, A.M., Rajakumar, A., Abouheif, E. 2020. Origin and elaboration of a major evolutionary transition in individuality. Nature 585, 239–244. (doi:10.1038/s41586-020-2653-6).
- Ramalho, M.de O., Kim, Z., Wang, S., Moreau, C.S. 2021. Wolbachia Across Social Insects: Patterns and Implications. Annals of the Entomological Society of America 114, 206–218 (doi:10.1093/aesa/saaa053).
- Rasheed, M.T., Bodlah, I., Fareen, A.G., Wachkoo, A.A., Huang, X., Akbar, S.A. 2019. A checklist of ants (Hymenoptera: Formicidae) in Pakistan. Sociobiology 66(3), 426-439 (doi:10.13102/sociobiology.v66i3.4330).
- Rasheed, S.B., Ali, M., Zaidi, F., Noreen, S. 2020. Diversity of ants (Hymenoptera: Formicidae) in residential area of Tarbela, Swabi: New recrds from Pakistan. The Journal of Animal and Plant Sciences 31: 617-624 (doi:10.36899/japs.2021.2.0250).
- Reyes, J.L. 2010. Apuntes sobre una comunidad de hormigas sinantrópicas en Santiago de Cuba (Hymenoptera: Formicidae). Cocuyo 18: 44-47.
- Roger, J. 1863b. Verzeichniss der Formiciden-Gattungen und Arten. Berl. Entomol. Z. 7(B Beilage: 1-65 (page 10, Combination in Prenolepis, Senior synonym of gracilescens)
- Rosas-Mejía, M., Guénard, B., Aguilar-Méndez, M. J., Ghilardi, A., Vásquez-Bolaños, M., Economo, E. P., Janda, M. 2021. Alien ants (Hymenoptera: Formicidae) in Mexico: the first database of records. Biological Invasions 23(6), 1669–1680 (doi:10.1007/s10530-020-02423-1).
- Sharaf, M. R., Wetterer, J. K., Mohamed, A. A., Aldawood, A. S. 2022. Faunal composition, diversity, and distribution of ants (Hymenoptera: Formicidae) of Dhofar Governorate, Oman, with updated list of the Omani species and remarks on zoogeography. European Journal of Taxonomy 838: 1-106 (doi:10.5852/ejt.2022.838.1925).
- Sharaf, M.R., Abdel-Dayem, M.S., Mohamed, A.A., Fisher, B.L., Aldawood, A.S. 2020. A preliminary synopsis of the ant fauna (Hymenoptera: Formicidae) of Qatar with remarks on the zoogeography. Annales Zoologici 70: 533-560 (doi:10.3161/00034541anz2020.70.4.005).
- Sharaf, M.R., Fisher, B.L., Collingwood, C.A., Aldawood, A.S. 2017. Ant fauna (Hymenoptera: Formicidae) of the Socotra Archipelago (Yemen): zoogeography, distribution and description of a new species. Journal of Natural History 51, 317–378 (DOI 10.1080/00222933.2016.1271157).
- Shiran, E., Mossadegh, M.S., Esfandiari, M. 2013. Mutualistic ants (Hymenoptera: Formicidae) associated with aphids in central and southwestern parts of Iran. Journal of Crop Protection 2: 1-12.
- Siddiqui, J. A., Li, J., Zou, X., Bodlah, I., Huang, X. 2019. Meta-analysis of the global diversity and spatial patterns of aphid-ant mutualistic relationships. Applied Ecology and Environmental Research 17: 5471-5524 (doi:10.15666/aeer/1703_54715524).
- Siddiqui, J.A., Bamisile, B.S., Khan, M.M., Islam, W., Hafeez, M., Bodlah, I., Xu, Y. 2021. Impact of invasive ant species on native fauna across similar habitats under global environmental changes. Environmental Science and Pollution Research 28(39), 54362–54382 (doi:10.1007/s11356-021-15961-5).
- Sinu P.A., Nasser, M., Rajan, P.D. 2006. Feeding fauna and foraging habits of tiger beetles found in agro-ecosystems in Western Ghats, India. Biotropica 38(4): 500-507 (doi:10.1111/j.1744-7429.2006.00174.x).
- Subedi, I.P., Budha, P.B., Bharti, H., Alonso, L. 2020. An updated checklist of Nepalese ants (Hymenoptera, Formicidae). ZooKeys 1006, 99–136 (doi:10.3897/zookeys.1006.58808).
- Taylor, B., Agoinon, N., Sinzogan, A., Adandonon, A., Kouaguou, Y. N., Bello, S., Wargui, R., Anato, F., Ouagoussounon, I., Houngbo, H., Tchibozo, S., Todjihounde, R., Vayssieres, J.F. 2018. Records of ants (Hymenoptera: Formicidae) from the Republic of Benin, with particular reference to the mango farm ecosystem. Journal of Insect Biodiversity 8(1): 6-29 (doi:10.12976/jib/2018.08.1.2).
- Trager, J. C. 1984b. A revision of the genus Paratrechina (Hymenoptera: Formicidae) of the continental United States. Sociobiology 9: 49-162 (page 153, see also)
- Tseng, S.-P. 2020. Evolutionary history of a global invasive ant, Paratrechina longicornis (Dissertation_全文 ). Ph.D. thesis, Kyoto University.
- Tseng, S.-P., Hsu, P.-W., Lee, C.-C., Wetterer, J.K., Hugel, S., Wu, L.-H., Lee, C.-Y., Yoshimura, T., Yang, C.-C.S. 2020. Evidence for common horizontal transmission of Wolbachia among ants and ant crickets: Kleptoparasitism added to the list. Microorganisms 8, 805. (doi:10.3390/MICROORGANISMS8060805).
- Ulysséa, M.A., Brandão, C.R.F. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia 57, 217–224 (doi:10.1590/s0085-56262013005000002).
- van Elst, T., Eriksson, T.H., Gadau, J., Johnson, R.A., Rabeling, C., Taylor, J.E., Borowiec, M.L. 2021. Comprehensive phylogeny of Myrmecocystus honey ants highlights cryptic diversity and infers evolution during aridification of the American Southwest. Molecular Phylogenetics and Evolution 155, 107036 (doi:10.1016/j.ympev.2020.107036).
- Vanderhaegen K, Naturinda Z, Kouakou LMM, Vanderheyden A, Dekoninck W (2019) First record of the invasive longhorn crazy ant, Paratrechina longicornis (Latreille, 1802) (Hymenoptera: Formicidae) from Mt. Elgon, eastern Uganda. BioInvasions Records 8: 505–514 (doi:10.3391/bir.2019.8.3.05).
- Varela-Hernández, F., Medel-Zosayas, B., Martínez-Luque, E.O., Jones, R.W., De la Mora, A. 2020. Biodiversity in central Mexico: Assessment of ants in a convergent region. Southwestern Entomologist 454: 673-686.
- Wang, C., Sung, P.-J., Lin, C.-C., Ito, F., Billen, J. 2023. Parthenogenetic reproduction in Strumigenys ants: An update. Insects 14, 195 (doi:10.3390/insects14020195).
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Wetterer, J. K.; Miller, S. E.; Wheeler, D. E.; Olson, C. A.; Polhemus, D. A.; Pitts, M.; Ashton, I. W.; Himler, A. G.; Yospin, M. M.; Helms, K. R.; Harken, E. L.; Gallaher, J.; Dunning, C. E.; Nelson, M.; Litsinger, J.; Southern, A.; Burgess, T. L. 1999. Ecological dominance by Paratrechina longicornis (Hymenoptera: Formicidae) an invasive tramp ant, in Biosphere 2. Florida Entomologist 82:381-388.
- Wetterer, J.K. 2008. Worldwide spread of the longhorn crazy ant, Paratrechina longicornis (Hymenoptera: Formicidae). Myrmecoloigcal News 11, 137-149.
- Wetterer, J.K. 2017. Invasive ants of Bermuda revisited. Journal of Hymenoptera Research 54, 33–41 (doi:10.3897/jhr.54.11444).
- Wetterer, J.K. 2021. Ants (Hymenoptera, Formicidae) of St. Vincent, West Indies. Sociobiology 68, e6725 (doi:10.13102/sociobiology.v68i2.6725).
- Wetterer, J.K., Espadaler, X., Ashmole, N.P., Mendel, H., Cutler, C., Endeman, J. 2007. Ants (Hymenoptera: Formicidae) of the South Atlantic islands of Ascension Island, St Helena, and Tristan da Cunha. Myrmecological News 10: 29-37.
- Wetterer, J.K., Wetterer, A.L. 2004. Ants (Hymenoptera: Formicidae) of Bermuda. Florida Entomologist 87(2), 212–221 (doi:10.1653/0015-4040(2004)087[0212:ahfob2.0.CO;2]).
- Wheeler, G. C.; Wheeler, J. 1986d. Supplementary studies on ant larvae: Formicinae (Hymenoptera: Formicidae). J. N. Y. Entomol. Soc. 94: 331-341 (page 336, larva described)
- Wheeler, W. M. 1921e. Chinese ants collected by Prof. C. W. Howard. Psyche (Camb.) 28: 110-115 (page 112, Combination in Paratrechina)
- Yamane, S., Tanaka, H.O., Hasimoto, Y., Ohashi, M., Meleng, P., Itioka, T. 2021. A list of ants from Lambir Hills National Park and its vicinity, with their biological information: Part II. Subfamilies Leptanillinae, Proceratiinae, Amblyoponinae, Ponerinae, Dorylinae, Dolichoderinae, Ectatomminae and Formicinae. Contributions from the Biological Laboratory, Kyoto University 31, 87–157.
- Yu, Y. 2016. Risk of alien species introduction to Ogasawara Islands : Case study of ants at Tokyo Port. World Heritage Studies 1, 86-89.
References based on Global Ant Biodiversity Informatics
- Arnold G. 1922. A monograph of the Formicidae of South Africa. Part V. Myrmicinae. Annals of the South African Museum 14: 579-674.
- Borowiec L., and S. Salata. 2018. Notes on ants (Hymenoptera: Formicidae) from Gambia (Western Africa). Annals of the Upper Silesian Museum in Bytom Entomology 26: 1-13.
- CSIRO Collection
- Diame L., B. Taylor, R. Blatrix, J. F. Vayssieres, J. Y. Rey, I. Grechi, and K. Diarra. 2017. A preliminary checklist of the ant (Hymenoptera, Formicidae) fauna of Senegal. Journal of Insect Biodiversity 5(15): 1-16.
- Eidmann H. 1944. Die Ameisenfauna von Fernando Poo. 27. Beitrag zu den Ergebnissen der Westafrika-Expedition. Zool. Jahrb. Abt. Syst. Ökol. Geogr. Tiere 76: 413-490.
- Emery, C. "Viaggio ad Assab nel Mar Rosso dei Signori G. Doria ed O. Beccari con il R. Avviso "Esploratore" dal 16 novembre 1879 al 26 febbraio 1880. I. Formiche." Annali del Museo Civico di Storia Naturale 16 (1881): 525-535.
- Forel A. 1901. Formiciden des Naturhistorischen Museums zu Hamburg. Neue Calyptomyrmex-, Dacryon-, Podomyrma- und Echinopla-Arten. Mitt. Naturhist. Mus. Hambg. 18: 43-82.
- Forel A. 1910. Ameisen aus der Kolonie Erythräa. Gesammelt von Prof. Dr. K. Escherich (nebst einigen in West-Abessinien von Herrn A. Ilg gesammelten Ameisen). Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 29: 243-274.
- Forel A. 1914. Formicides d'Afrique et d'Amérique nouveaux ou peu connus. Bulletin de la Société Vaudoise des Sciences Naturelles 50: 211-288.
- Garcia F.H., Wiesel E. and Fischer G. 2013.The Ants of Kenya (Hymenoptera: Formicidae)Faunal Overview, First Species Checklist, Bibliography, Accounts for All Genera, and Discussion on Taxonomy and Zoogeography. Journal of East African Natural History, 101(2): 127-222
- IZIKO South Africa Museum Collection
- Karavaiev V. 1911. Ameisen aus Aegypten und dem Sudan. Rus. Entomol. Obozr. 11: 1-12.
- Kouakou L. M. 2015. Evaluation de la diversite des especes de fourmis anthropophiles, natives, exotiques et potentielles invasives en Cote d'Ivoire. Royal Belgian Institute of Natural Sciences
- Kouakou L. M. M., K. Yeo, K. Ouattara, W. Dekoninck, T. Delsinne, and S. Konate. 2018. Investigating urban ant community (Hymenoptera: Formicidae) in port cities and in major towns along the border in Côte d’Ivoire: a rapid assessment to detect potential introduced invasive ant species. Journal of Animal and Plant Sciences 36(1): 5793-5811.
- Kouakou L. M. M., W. Dekoninck, M. Kone, T. Delsinne, K. Yeo, K. Ouattara, and S. Konate. 2018. Diversity and distribution of introduced and potentially invasive ant species from the three main ecoregions of Côte d’Ivoire (West Africa). Belgian Journal of Zoology 148 (1): 83–103.
- Madl M. 2019. Notes on the ant fauna of Eritrea (Insecta: Hymenoptera: Formicidae): type specimens deposited in the Natural History Museum Vienna (Austria) and a preliminary checklist. Ann. Naturhist. Mus. Wien, B 121: 9-18.
- Magboul R., A. Khider, E. Idris, and T. H. Alam. 2013. Ant diversity at Sunut forest, Khartoum, Sudan. Egypt. Acad. J. Biolog. Sci. 6(1): 43-46.
- Medler J. T. 1980: Insects of Nigeria - Check list and bibliography. Mem. Amer. Ent. Inst. 30: i-vii, 1-919.
- Menozzi C. 1926. Formiche dell'Africa centrale. Bollettino della Società Entomologica Italiana. 58: 36-41.
- Menozzi C. 1930. Formiche della Somalia italiana meridionale. Memorie della Società Entomologica Italiana. 9: 76-130.
- Menozzi C. 1942. Formiche dell'isola Fernando Poo e del territorio del Rio Muni (Guinea Spagnola). 24. Beitrag zu den wissenschaftlichen Ergebnissen der Forschungsreise H. Eidmann nach Spanisch-Guinea 1939 bis 1940. Zoologischer Anzeiger 140: 164-182.
- Mikissa J. B., J. H. C. Delabie, J. L. Mercier, and D. Fresnau. 2008. Preliminary Assessment on the Interactions of Wasmannia auropunctata in Native Ant Communities (Hymenoptera: Formicidae) of a Mosaic Gallery Forest/Savannah in Lope National Park, Gabon. Sociobiology 51(1): 207-218.
- Santschi F. 1914. Formicides de l'Afrique occidentale et australe du voyage de Mr. le Professeur F. Silvestri. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 8: 309-385.
- Santschi F. 1920. Études sur les maladies et les parasites du cacaoyer et d'autres plantes cultivées à S. Thomé. X. Fourmis de S. Thomé. Extrait des Mémoires publiés par la Société Portugaise des Sciences Naturelles. Lisbonne: Imprimerie de la Librairie Ferin, 4 pp.
- Santschi F. 1935. Hymenoptera. I. Formicidae. Mission Scientifique de l'Omo 2: 255-277.
- Stitz H. 1916. Formiciden. Ergebnisse der Zweiten Deutschen Zentral-Afrika Expedition 1: 369-405.
- Taylor B., N. Agoinon, A. Sinzogan, A. Adandonon, Y. N'Da Kouagou, S. Bello, R. Wargui, F. Anato, I. Ouagoussounon, H. Houngbo, S. Tchibozo, R. Todjhounde, and J. F. Vayssieres. 2018. Records of ants (Hymenoptera: Formicidae) from the Republic of Benin, with particular reference to the mango farm ecosystem. Journal of Insect Biodiversity 8(1): 006–029.
- Vanderhaegen K., Z. Naturinda, L. M. M. Kouakou, A. Vanderheyden, and W. Dekoninck. 2019. First record of the invasive longhorn crazy ant, Paratrechina longicornis (Latreille, 1802) (Hymenoptera: Formicidae) from Mt. Elgon, eastern Uganda. BioInvasions Records 8.
- Wetterer J. K., X. Espadaler, A. L. Wetterer, D. Aguin-Pombo, and A. M. Franquinho-Aguiar. 2006. Long-term impact of exotic ants on the native ants of Madeira. Ecological Entomology 31: 358-368.
- Wetterer J. K., X. Espadaler, A. L. Wetterer, D. Aguin-Pombo, and A. M. Franquinho-Aguiar. 2007. Ants (Hymenoptera: Formicidae) of the Madeiran archipelago. Sociobiology 49: 265-297.
- Wheeler W. M. 1922. Ants of the American Museum Congo expedition. A contribution to the myrmecology of Africa. II. The ants collected by the American Museum Congo Expedition. Bulletin of the American Museum of Natural History 45: 39-269.
- Wheeler W. M. 1922. Ants of the American Museum Congo expedition. A contribution to the myrmecology of Africa. VIII. A synonymic list of the ants of the Ethiopian region. Bulletin of the American Museum of Natural History 45: 711-1004
- Yeo K., L. M. M. Kouakou, W. Dekoninck, K. Ouattara, and S. Konate. 2016. Detecting intruders: assessment of the anthropophilic ant fauna (Hymenoptera: Formicidae) in the city of Abidjan and along access roads in Banco National Park (Côte d’Ivoire). Journal of Entomology and Zoological Studies 4(4): 351-359.
- Yeo K., T. Delsinne, S. Komate, L. L. Alonso, D. Aidara, and C. Peeters. 2016. Diversity and distribution of ant assemblages above and below ground in a West African forest–savannah mosaic (Lamto, Cote d’Ivoire). Insectes Sociaux DOI 10.1007/s00040-016-0527-6
- Yeo K., and A. Hormenyo. 2007. A Rapid Survey of Ants in Ajenjua Bepo and Mamang River Forest Reserves, Eastern Region of Ghana. Pp 27-29. In McCullough, J., P. Hoke, P. Naskrecki, and Y. Osei-Owusu (eds.). 2008. A Rapid Biological Assessment of the Ajenjua Bepo and Mamang River Forest Reserves, Ghana. RAP Bulletin of Biological Assessment 50. Conservation International, Arlington, VA, USA.
- Pages using DynamicPageList3 parser function
- Common Name
- Highly invasive
- Polygynous
- Supercolonies
- Parthenogenetic
- Photo Gallery
- Tropical
- South subtropical
- Nesting Notes
- FlightMonth
- Aphid Associate
- Host of Aphis coreopsidis
- Host of Aphis craccivora
- Host of Aphis gossypii
- Cricket Associate
- Host of Myrmecophilus americanus
- Tiger beetle Associate
- Host of Cicindela duponti
- Fungus Associate
- Host of Absidia corymbifera
- Host of Acremonium sp.
- Host of Aspergillus candidus
- Host of Aspergillus ochraceus
- Host of Aspergillus versicolor
- Host of Cladosporium sp.
- Host of Cunninghamella equinalata
- Host of Cunninghamella polymorpha
- Host of Geotrichum sp.
- Host of Penicillium sp.
- Host of Purpureocillium lilacinum
- Host of Aspergillus flavus
- Host of Metarhizium anisopliae
- Karyotype
- Species
- Extant species
- Formicidae
- Formicinae
- Lasiini
- Paratrechina
- Paratrechina longicornis
- Formicinae species
- Lasiini species
- Paratrechina species
- Ssr




