Acromyrmex charruanus
| Acromyrmex charruanus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Acromyrmex |
| Species: | A. charruanus |
| Binomial name | |
| Acromyrmex charruanus Rabeling, Schultz, Bacci & Bollazzi, 2015 | |
The parasitic queens of Acromyrmex charruanus are known to be inquilines in the nests of Acromyrmex heyeri in Uruguay. Despite Acromyrmex charruanus being known from only two collections, significant strides were made in studying the biology of this species when it was discovered (see Rabeling et al. (2015), summarised below).
| At a Glance | • Workerless Inquiline |
Identification
Rabeling et al. (2015) - Acromyrmex charruanus is a social parasite of the leaf-cutting ant Acromyrmex heyeri and does not exhibit the morphological adaptations and/or reductions that are observed in Pseudoatta argentina, Mycocepurus castrator, and a hitherto undescribed species of Acromyrmex social parasite from Bahia in Brazil. Notwithstanding, morphologically the gyne of A. charruanus can easily be distinguished from the host gyne by its smaller size, darker color, and abundance of recurved, coarse setae. Relative to its smaller body size in comparison to the host, A. charruanus is also characterized by longer appendages, mandibles, and antennal scapes, as well as a shorter gaster. In the field, A. charruanus can be distinguished from its host by the significantly smaller size and the presence of alate females and males in the nest during the austral fall (February), whereas A. heyeri’s mating season typically occurs during the austral spring (October–December; see below).
The male of A. charruanus is not gynandromorphic and can easily be distinguished from the host male by its smaller size and the presence of dark, long, distinctly coarse, semierect setae, instead of the short, yellow, appressed setae found in the host males. The head of the parasite male is quadrate, not wider than long (as in A. heyeri), and the parasite’s mandibles are slender, bearing a variable number of teeth and indistinct denticles, in contrast to the broadly triangular mandibles of the host. The parasite’s genitalia are smaller than the host’s genitalia (paramere length: A. heyeri = 1.5 mm, A. charruanus = 0.9 mm; aedeagus length: A. heyeri = 0.33 mm, A. charruanus = 0.23 mm) and the ventral border of the aedeagus bears 12 teeth instead of 14, as in A. heyeri.
Interestingly, both gynes and males of A. charruanus are morphologically similar to a potentially distantly related Acromyrmex species, the social parasite Acromyrmex ameliae from Brazil, which presumably evolved convergently due to similar selective pressures related to the parasitic lifestyle. Gynes of A. charruanus can be distinguished from those of A. ameliae by their slightly smaller size, and, notably, by their long setae, which are recurved and appressed, instead of being erect or semi-erect, as is the case in A. ameliae. In addition, A. ameliae gynes are darker in color and the rugoreticulations on the head, meso-, and metasoma are more strongly developed than they are in A. charruanus. Males of A. charruanus are significantly smaller than A. ameliae males and are darker in color, the spines and ridges on the petiole and postpetiole are less pronounced, the integumental sculpturing is less developed, and the propodeal spines are shorter. Geographic distribution is also indicative of species identity, unless the discovery of additional populations markedly alters the distribution of either species. So far, A. charruanus is only known from central Uruguay and A. ameliae is only known from the Brazilian state of Minas Gerais.
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: -33.9042° to -33.9042°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Uruguay (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
|
Rabeling et al. (2015) - Our conclusions about the life history and biology of A. charruanus are based on observations made on two parasitized A. heyeri colonies. Further attempts to find this social parasite have so far failed. Therefore, our observations should be regarded as initial insights into the parasite’s biology and life history. Other aspects of its behavioral ecology remain unknown and further field studies and laboratory experiments are required.
Diametrically opposed mating seasons of host and parasite: the chronologies of the mating seasons of host and parasite were observed to be diametrically opposed to one another. Winged gynes and males of A. charruanus were present in their host colonies during the southern hemisphere fall (i.e., February) and at this time no sexual offspring of the host species, A. heyeri, were observed. The mating flights of A. heyeri, and of all other Acromyrmex species, occur during the spring months, October to December, in Uruguay, southern Brazil, and northern Argentina (Bonetto 1959; Zolessi and Abenante 1974; Zolessi and Gonzalez 1974, 1979; Diehl-Fleig 1993). Therefore, sexual offspring of host and parasite are separated by a time window of at least 2 months.
Nuptial flights and mating locality: we did not observe fully developed mass nuptial flights of A. charruanus in the field. However, upon opening the thatch mound of one parasitized A. heyeri nest, alate reproductives of A. charruanus immediately started to fly. This behavior was observed repeatedly in laboratory colonies of A. charruanus in which reproductives attempted to fly as soon as the nest boxes were opened. In contrast, the attine inquiline social parasites Pseudoatta argentina and Mycocepurus castrator were never observed to fly and may in fact be incapable of doing so (Bruch 1928; Gallardo 1929; Rabeling and Bacci 2010). In addition, A. charruanus reproductives were never observed to copulate inside lab colonies, which suggests that A. charruanus performs regular mating flights and mates in midair or on the ground, as has been observed in non-parasitic leaf-cutting ant species (Diehl-Fleig 1993; Johnson and Rissing 1993). In contrast, both P. argentina and M. castrator are adelphogamous and mate inside the host colony (Bruch 1928; Gallardo 1929; Rabeling and Bacci 2010).
Reproductive strategy: initial observations suggest that A. charruanus reproduces semelparously. We marked the nests of two parasitized A. heyeri colonies at Plantacion Cruz Roja in February and upon revisiting the same nests in May of the same year, both nests were empty and the thatch mounds had collapsed, suggesting that the colonies had died. We also observed that the fungus gardens of parasitized A. heyeri colonies looked tattered while parasite alates were present in the nest and that the amount of fungus garden was reduced in comparison to unparasitized A. heyeri colonies; the fungal biomass was not quantified, however. Therefore, it seems possible that the host colony is incapable of recovering from the mass rearing of the parasitic brood and dies after the mass exodus of parasite alates. Alternatively, A. charruanus queens could preferentially exploit old host colonies that have lost the host queen.
Parasitism rate: to estimate the rate at which colonies of Acromyrmex heyeri were parasitized by A. charruanus we opened 100 nests of A. heyeri during our fall survey (February), when alates of the parasite were present, and found only two A. heyeri colonies containing A. charruanus alates. During the spring survey (November) we opened 100 nests of the same population and did not find any parasites. Therefore, parasites are probably best detected prior to their mass nuptial flights and likely escaped detection when only the parasite queen(s), and not the alate parasite reproductive offspring, were present. To calculate the approximate rate of parasitism we used only the colony counts from the fall survey, suggesting that in this Central Uruguayan population A. charruanus parasitizes A. heyeri colonies at a rate of approximately 2 %. It is possible that parasites escaped detection in some of the colonies we examined, and therefore our estimated rate of parasitism should be regarded as a minimum estimate. The parasitism rates of the hitherto undescribed social parasite from Bahia, of A. insinuator, and of A. ameliae were estimated to be 8, 40, and 70 %, respectively (Delabie et al. 1993; Bekkevold and Boomsma 2000; De Souza et al. 2007).
Host nest density: in Uruguay, northern Argentina, and southern Brazil A. heyeri is known to build large dome-shaped thatch-mound nests (Bollazzi et al. 2008). In each season, we opened 100 A. heyeri thatch-mound nests distributed over an area of roughly 30 hectares. Accordingly, the nest density of A. heyeri was approximately 3–4 colonies per hectare. We did not open every colony we detected at our field site and almost certainly missed some small, incipient colonies in wooded areas. Therefore, this estimate of 3–4 colonies per hectare should be regarded as a minimum estimate of the actual nest density of A. heyeri in this habitat.
The morphological, behavioral, and natural history data indicate that Acromyrmex charruanus is a permanent and obligate inquiline social parasite of the thatch-mound-building, leaf-cutting ant Acromyrmex heyeri. We never encountered free-living colonies of A. charruanus and the parasite seems to rely on the host colony for successful reproduction. The presence of female host brood (i.e., worker brood) in the parasitized A. heyeri colonies suggests that A. charruanus is a queen-tolerant inquiline parasite (Buschinger 1986, 2009; Holldobler and Wilson 1990; Bourke and Franks 1991).
Castes
Male
 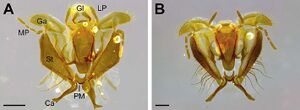 
| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- charruanus. Acromyrmex charruanus Rabeling, et al. 2015: 338, figs. 1a,c,e, 2a,c,e, 3a, 4a,c (q.m.) URUGUAY.
- Type-material: holotype queen, 80 paratype queens, 125 paratype males.
- Type-locality: holotype Uruguay: Florida Dept., 6 km. SW Cerro Colorado, Eucalyptus globulus plantation, “Plantación Cruz Roja”, 33.9042°S, 55.59418°W, 224 m., 25.ii.2013, CR130225-03 (Bollazzi & C. Rabeling); paratypes: 73 queens, 119 males with same data but 27.ii.2013, CR130227-31, 7 queens, 6 males (recorded separately but apparently with same data as last).
- Type-depositories: MZSP (holotype); CPDC, CRPC, MBPC, MZSP, USNM (paratypes).
- Distribution: Uruguay.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Queen
Holotype. TL 6.92, WL 2.08, HL 1.21, HW 1.33, IOD 1.54, ML 0.92, FLD 0.74, SL 1.28, EL 0.31, EW 0.26, PrW 1.1, FL 2.03, PL 0.51, PW 0.41, PPL 0.41, PPW 0.74, GL 1.79, CI 111, MI 77, SI 96. Paratype (n = 10): TL 6.64–7.23,WL 2.03–2.15, HL 1.15–1.28, HW 1.28–1.36, IOD 1.46–1.59, ML 0.9–0.95, FLD 0.74–0.77, SL 1.26–1.31, EL 0.28–0.31, EW 0.23–0.28, PrW 1.1–1.21, FL 1.92–2.08, PL 0.46–0.54, PW 0.41–0.46, PPL 0.38–0.46, PPW 0.72–0.77, GL 1.67–1.97, CI 106–113, MI 72–80, SI 94–100.
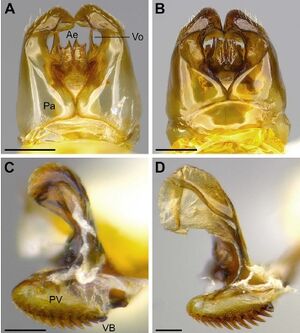
One of the smallest Acromyrmex leaf-cutting ant species known (WL 2.08; TL 6.92), with long mandibles (MI 77) and appendages (FL 2.03; SI 96) relative to body size. Integument with microscopic honey-comb pattern, which becomes visible at 409 magnification and higher; sculpturing of head, meso-, and metasoma coarsely granulate with distinct rugae and tubercles; integument with oily or waxy sheen. Body surface covered with long, appressed, and distinctly coarse setae. Color: shades of brown, variable from yellowish to reddish to dark brown; mandibles distinctly lighter in color, dark orange; ridges of rugae and tips of tubercles black; dorsum of mesosoma dark brown, with black markings. Head: head shape trapezoidal, slightly wider than long (CI 111); sides moderately tapering anteriorly between the eyes and mandibular insertion; head size small relative to mesosoma. Mandible broadly triangular with a distinct apical and preapical tooth, followed by seven smaller teeth, some of which are interspersed by even smaller denticles; mandible surface smooth and distinctly shiny. Palp formula 4:2, the plesiomorphic condition of fungus-growing ants. Posterior margin of clypeus trapezoidal, broadly inserted between frontal lobes; anterior margin of clypeus shiny and median portion concave. Unpaired median clypeal seta thin and short (0.15), only projecting over the anterior clypeal margin by half its length. Frontal lobe broadly rounded, fully covering the condylar bulb in full-face view; lateral margin of frontal lobe serrate with two distinct tooth-like projections. Frontal carina extending towards the posterolateral corner of the head. Preocular carina forming a straight line in lateral view and traversing the area of the antennal scrobe by one third of the scrobe’s width. Eyes large (EL 0.31, EW 0.26) and strongly convex. In contrast, the three ocelli are small and embedded in the integument. Antennae with 11 segments. Antennal scape long (SL 1.28) with abundant, appressed setae, surpassing the posterior margin of the head by one third of its length. Mesosoma: Mesosoma slender with caste-specific modifications related to wing bearing. Dorsolateral pronotal spine long, slender, and sharply pointed in dorsal view. Ventrolateral pronotal spine narrowly triangular and sharply pointed, not curved. Dorsum of mesosoma covered with longitudinal and reticulate rugulae. Posterior margin of scutellum concave and bidentate in dorsal view; teeth wide at base, forming a broad, almost 90° angle. Bulla and meatus of metapleural gland not notably modified from the condition in the host species. Propodeal spines straight, long, slender, and sharply pointed, projecting away from the propodeum at a 90° angle in lateral view. Metasoma: anterior peduncle of petiole short, about one fourth the length of the petiolar node. Dorsum of petiolar node with a pair of short teeth, almost as wide at their bases as they are high. Postpetiole wider than long in dorsal view (PPW 0.74; PPL 0.41), posterior margin straight. Gaster short (GL 1.79). First gastric tergite notably tuberculate, covered with abundant recurved setae. Except for the smaller size, forewing and hindwing resembling the wings of Acromyrmex heyeri.
Male
Paratype (n = 10): TL 6.64–7.23, WL 2.03–2.15, HL 1.15–1.28, HW 1.28–1.36, IOD 1.46–1.59, ML 0.9–0.95, FLD 0.74–0.77, SL 1.26–1.31, EL 0.28–0.31, EW 0.23–0.28, PrW 1.1–1.21, FL 1.92–2.08, PL 0.46–0.54, PW 0.41–0.46, PPL 0.38–0.46, PPW 0.72–0.77, GL 1.67–1.97, CI 106–113, MI 72–80, SI 94–100. A small male (WL 2.03–2.28; TL 6.54–7.03), smaller than any other Acromyrmex male. Integument with microscopic honeycomb pattern; sculpturing of head and mesosoma finely granulate; posterior half of the head with fine rugulae; metasoma smooth and shiny. Body surface covered with long, dark, both semi-erect and appressed, distinctly coarse setae. Color: head and mesosoma black; appendages and metasoma dark brown; anterior half of antennal flagellum and mandibles light brown. Head: approximately as wide as long (CI 97–106); sides subparallel behind the level of the eye, tapering anteriorly towards the mandibular insertion; head size small relative to mesosoma. Mandible narrowly triangular, slender, with a distinct apical and preapical tooth, followed by two to three smaller teeth, which are interspersed by tiny denticles; mandible surface smooth, shiny. Palp formula 4:2. Clypeus and unpaired clypeal seta as in gyne. Frontal lobe narrow, leaving the anterior half of the condylar bulb exposed in full-face view. Preocular carina indistinct, traversing the area of the antennal scrobe by half the scrobe’s width. Eyes large (EL 0.33–0.38, EW 0.31–0.33) and strongly convex. Ocelli large and raised above the surface of the head. Antennae with 13 segments. Antennal scape long (SL 1.13–1.26) with appressed setae, surpassing the posterior margin of the head by half its length. Mesosoma: Mesosoma relatively slender with sexspecific modifications related to wing bearing. Dorsolateral pronotal spine short, triangular, and sharply pointed in dorsal view. Ventrolateral pronotal spine broadly triangular, with broad tip, not curved. Dorsum of mesosoma with fine longitudinal rugulae. Scutellum as in gyne. Bulla and meatus of metapleural gland small, not notably modified from the condition in A. heyeri. Propodeal spines narrowly triangular. Orifice of propodeal spiracle round in lateral view, not slit-shaped. Orifice of metapleural gland oval, pointing dorso-ventrally; shape not notably modified from metapleural gland opening in A. heyeri. Metasoma: petiole and postpetiole as in gyne. Gaster slender: first gastric tergite smooth, shiny, and covered with abundant semi-erect setae. Genitalia: in toto, excluding the basal ring, parameres slightly longer (0.9) than wide (0.85); apical lobe of paramere evenly rounded with less than 10 long, erect setae. In lateral view, aedeagus small (0.23), ventral border of penis valve bearing 12 recurved teeth, the anterior three of which are small and weakly sclerotized, whereas the posterior nine are distinctly larger and heavily sclerotized, as notable by the darker brown coloration.
Type Material
Holotype: alate gyne, URUGUAY, Florida Department, 6 km SW of Cerro Colorado, Eucalyptus globulus plantation ‘‘Plantacion Cruz Roja’’, GPS coordinates: 33°54′15″S 55°35′39″W / 33.9042°S 55.59418°W elevation 224 m above sea level, collection date: 25 February 2013, col. Bollazzi & Rabeling; collection code: CR130225-03, ex Acromyrmex heyeri nest. The holotype is deposited at the MZSP and carries the unique specimen identifier No. USNMENT00758784. Paratypes: same data as holotype, 73 alate gynes and 119 males, USNMENT00758785-00758977. Same data as holotype, but ‘‘27 February 2013, collection code: CR130227-31’’, 7 alate gynes and 6 males, USNMENT00758778-00758990.
Etymology
The species epithet ‘‘charruanus’’ refers to ‘‘Charrua’’, the name given to the indigenous people of Uruguay by Europeans, who arrived in the sixteenth century. Colloquially, the Uruguayan people also refer to themselves as ‘‘charruas’’. Therefore, and in allusion to the etymology of the enigmatic social parasite Pseudoatta argentina, the species name refers to the geographic region where this social parasite was discovered.
References
- Cantone S. 2018. Winged Ants, The queen. Dichotomous key to genera of winged female ants in the World. The Wings of Ants: morphological and systematic relationships (self-published).
- Dahan, R.A., Grove, N.K., Bollazzi, M., Gerstner, B.P., Rabeling, C. 2021. Decoupled evolution of mating biology and social structure in Acromyrmex leaf-cutting ants. Behavioral Ecology and Sociobiology 76, 7 (doi:10.1007/s00265-021-03113-1).
- de la Mora, A., Sankovitz, M., Purcell, J. 2020. Ants (Hymenoptera: Formicidae) as host and intruder: recent advances and future directions in the study of exploitative strategies. Myrmecological News 30: 53-71 (doi:10.25849/MYRMECOL.NEWS_030:053).
- Rabeling, C.; Schultz, T. R.; Bacci, M., Jr.; Bollazzi, M. 2015. Acromyrmex charruanus: a new inquiline social parasite species of leaf-cutting ants. Insectes Sociaux. 62:335–349. doi:10.1007/s00040-015-0406-6

