Acromyrmex ameliae
| Acromyrmex ameliae | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Acromyrmex |
| Species: | A. ameliae |
| Binomial name | |
| Acromyrmex ameliae De Souza, Soares & Della Lucia, 2007 | |
A social parasite of Acromyrmex brunneus and Acromyrmex subterraneus.
| At a Glance | • Inquiline |
Identification
De Souza et al. (2007) - Acromyrmex ameliae is a social parasite with much smaller reproductives (females and males) than those of its hosts. Morphometrically, the A. ameliae queen is not a simple miniature of its hosts’ queens, like Myrmica microrubra and its host Myrmica rubra (Steiner et al., 2005). Here, we can distinguish the new species from the other of the group with propodeal spines: they are straight and laterally compressed unlike Acromyrmex subterraneus where they are slight to strongly curved and conical. Acromyrmex ameliae differs from Acromyrmex insinuator not only by its size and color (brown dark against yellowish-orange) but as well it does not present a single strong median ruga extending from the central ocellus to the level of the posterior borders of lateral ocelli, like A. insinuator. On the contrary, around its central ocellus, the cuticle is wholly rugous without a distinct median ruga. In A. insinuator the anteroventral edge of the postpetiole is broadly and evenly concave, without a broad median anteroventral extension. The anteroventral portion of the post-petiole in A. ameliae has irregular extensions, without the concavity present in the first species. As in Acromyrmex insinuator, reproductives (females and males) A. ameliae very much resemble the host species, although there has been a pronounced reduction in body size
Distribution
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
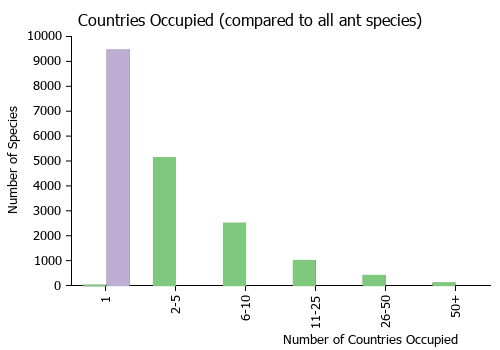
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
|
This ant is a social parasite in the nests of Acromyrmex subterraneus. The form of Social Parasitism is an inquiline with queens and workers smaller than their host.
De Souza et al. (2007) - There are previous reports on polygyny in the two host subspecies (Della Lucia & Vilela, 1989; Delabie, 1989). During nest collection, we indeed found three monoginic parasitized nests which had 2, 3 and 4 queens of A. ameliae. The first collection of the parasite was in October, 2003, when two nests were collected with hundreds of alate males and queens of the parasite. In April of the following year, we again collected nests with the alate parasites. This suggests that the production of the reproductive caste in A. ameliae may occur throughout the year. In the laboratory, males and queens flew towards the light, indicating that this species is likely to perform the nuptial flight in nature. As in A. insinuator, Acromyrmex ameliae produce a workforce. This seems to be essential for the production of the parasite alates (Sumner et al., 2003), but this trait is being selected against over evolutionary time, although it has not yet been lost. We need to investigate if the host cares for the parasite alates. In this case, parasite workers may not be needed.
We found alate parasites in two different seasons (April and October), unlike the host species, which has only a single synchronized nuptial flight per year in November and December. More than one nuptial flight each year could increase their likelihood of successful invasion of new colonies. The well defined nuptial flight of the hosts is normally observed in November and December so that newly fertilized parasite queens (produced in April) can colonize established colonies of A. subterraneus subterraneus well before they reproduce themselves.
We excavated 14 colonies of A. subterraneus and found all of them parasitized by A. ameliae. Thus, A. ameliae appears to be very common, yet always overlooked in the past.
Castes
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- ameliae. Acromyrmex ameliae De Souza, Soares & Della Lucia, 2007: 252, figs. 1, 3, 5, 7, 9-14 (w.q.m.) BRAZIL (Minas Gerais).
- Type-material: holotype queen, 18 paratype workers, 20 paratype queens, 21 paratype males.
- Type-locality: holotype Brazil: Minas Gerais, Paraopeba, 6.x.2003 (D.J. Souza); paratypes: 1 male with same data, 18 workers, 20 queens, 20 males with same data but 20.iv.2004 (I.M.F. Soares).
- Type-depositories: MZSP (holotype); CPDC, LECV, MZSP (paratypes).
- Distribution: Brazil.
Type Material
Holotype queen labeled ‘Brazil: Paraopeba MG/06 Oct 2003/ D. J. Souza’(MZUSP). Paratypes 20 queens, 20 males and 18 workers. Labeled ‘Brazil: Paraopeba MG/ 20 April 2004/ I. M. F. Soares (MZUSP). Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
HL = 0.6; HW = 0.7; ML = 0.3; WL = 0.9; SL = 0.8; ED = 0.1.
We verified that the distance from spiracle to bulla relative to pronotum width differed significantly among the minor workers of host and parasite (F1, 298 = 551.36, P < 0.01). Two groups are clearly shown in Figure 15: one had a small number (n = 25) of parasite workers and another formed by a large number of host minor workers (n = 275). The fact that the workers sorted into two groups, as well as morphological differences between the groups, is highly suggestive. We found some A. ameliae workers are larger than host minors but this is because larger host minors were not sampled. Preliminary genetic analysis by RAPD (Random amplified polymorphic DNA) markers clearly shows differences between the two groups, confirming these results. As in Acromyrmex insinuator, the workers of A. ameliae have a significantly smaller distance from their spiracle to bulla than their host minor workers of same pronotum width (Figs. 16 and 17). These results are almost identical to those obtained by Sumner et al. (2003) for A. insinuator.
Queen
Holotype: HL = 1.5; HW = 1.4; ML = 0.7; WL = 2.6; SL = 1.5; ED = 0.4.
This species has a palpal formula of 4, 2 and 11 antennal segments as is typical for attine ants. Acromyrmex ameliae queens have large and convex eyes and the inferior pronotal spines are straight and forward-positioned as in the host species. The color of the analyzed parasite queens vary from brownish to brownish-black. The queens of A. ameliae are much smaller than those of its hosts, with a WL ~ 0.6 as great. Acromyrmex ameliae further has a more abundant pilosity with thicker and longer hairs on the gaster, on the dorsal portion of the alitrunk and on the anterior portion of the head, in comparison to that on the host subspecies. The parasite queen also has prominent ridges on the head and on the first segment of the gaster and expansions on the anteroventral margin of the postpetiole that are not seen on the host subspecies. The tubercles on the gaster of A. ameliae are more or less ordinated in four longitudinal lines similar to the host species. However, these tubercles are very much reduced and less prominent when compared to those of the hosts.
Male
Paratype: HL = 1.0; HW = 0.9; ML = 0.6; WL = 2.2; SL = 1.3; ED = 0.4.
The males of A. ameliae have 13 antennal segments. This characteristic was not constant since five individuals seemed to have 12 segments as a consequence of the fusion of segments 4 and 5 of the antennal funiculum like in the host males. This fact was also observed by Schultz et al. (1998) in Acromyrmex insinuator. Males of A. ameliae are visually smaller than the males of the studied host species (about 1.2 times). The antenna has a color gradient which ranges from dark brown to dark yellow when going from tip to base. The color of the males as well as of the queens is close to that of the host subspecies Acromyrmex subterraneus, that is, very dark brown independent of the parasitized subspecies. However, as pointed out by Gonçalves (1961), the character color shows variation even inside the same nest. Newly emerged males and queens of A. ameliae of a lighter color were observed by the authors in the nests, but they became dark brown after a few days had elapsed. Their mandibles have a terminal tooth greater than the other teeth, which vary from 5 to 7. The ventral portion of the post-petiole of the parasite has irregular projections that are not seen in the host whose petiole margin is more regular and presents a concavity not observed on the parasite. Ridges and tubercles can be observed on the gaster of A. ameliae males, but these are missing in the host species whose gaster is smooth and shiny.
Etymology
This species is named after Amélia Maria de Souza, mother of the first author of this work.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- 2n = 36, karyotype = 10M +16SM + 8ST + 2A (Brazil) (Barros et al., 2021; Cardoso et al. 2018; de Castro et al., 2020; Micolino et al., 2021).
References
- Barros, L.A.C., Aguiar, H.J.A.C., Teixeira, G.C., Souza, D.J., Delabie, J.H.C., Mariano, C.S.F. 2021. Cytogenetic studies on the social parasite Acromyrmex ameliae (Formicidae: Myrmicinae: Attini) and its hosts reveal chromosome fusion in Acromyrmex. Zoologischer Anzeiger 293, 273–281 (doi:10.1016/j.jcz.2021.06.012).
- Buschinger, A. 2009. Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecological News 12: 219-235.
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Cantone S. 2018. Winged Ants, The queen. Dichotomous key to genera of winged female ants in the World. The Wings of Ants: morphological and systematic relationships (self-published).
- Cardoso, D. C., Cristiano, M. P. 2021. Karyotype diversity, mode, and tempo of the chromosomal evolution of Attina (Formicidae: Myrmicinae: Attini): Is there an upper limit to chromosome number? Insects 1212, 1084 (doi:10.3390/insects12121084).
- Cristiano, M.P., Cardoso, D.C., Sandoval‐Gómez, V.E., Simões‐Gomes, F.C. 2020. Amoimyrmex Cristiano, Cardoso, Sandoval, gen. nov. (Hymenoptera: Formicidae): a new genus of leaf‐cutting ants revealed by multilocus molecular phylogenetic and morphological analyses. Austral Entomology 59, 643–676 (doi:10.1111/aen.12493).
- de Castro, C.P.M., Cardoso, D.C., Micolino, R., Cristiano, M.P. 2020. Comparative FISH-mapping of TTAGG telomeric sequences to the chromosomes of leafcutter ants (Formicidae, Myrmicinae): is the insect canonical sequence conserved? Comparative Cytogenetics 14(3): 369–385 (doi:10.3897/CompCytogen.v14i3.52726).
- de la Mora, A., Sankovitz, M., Purcell, J. 2020. Ants (Hymenoptera: Formicidae) as host and intruder: recent advances and future directions in the study of exploitative strategies. Myrmecological News 30: 53-71 (doi:10.25849/MYRMECOL.NEWS_030:053).
- De Souza, D. J., I. M. F. Soares and T. M. C. Della Lucia. 2007. Acromyrmex ameliae sp. n. (Hymenoptera: Formicidae): A new social parasite of leaf-cutting ants in Brazil. Insect Science. 14:251-257.
- Farder-Gomes, C.F., Oliveira, M.A., Castro Della Lucia, T.M., Serrão, J.E. 2019. Morphology of the ovary and spermatheca of the leafcutter ant Acromyrmex rugosus queens (Hymenoptera: Formicidae). Florida Entomologist 102, 515-519 (doi:10.1653/024.102.0312).
- Micolino, R., Baldez, B.C.L., Sánchez-Restrepo, A.F., Calcaterra, L., Cristiano, M.P., Cardoso, D.C. 2021. Karyotype structure and cytogenetic markers of Amoimyrmex bruchi and Amoimyrmex silvestrii: contribution to understanding leaf-cutting ant relationships. Genome 651, 43–51 (doi:10.1139/gen-2021-0044).
- Pikart, T. G., P. G. Lemes, W. C. d. C. Morais, J. C. Zanuncio, and T. M. C. Della Lucia. 2015. Recognition and Aggression of conspecific and heterospecific worker in Acromyrmex subterraneus subterraneus (Forel) (Hymenoptera: Formicidae). Sociobiology. 62:28-33. doi:sociobiology.v62i1.28-33 10.13102 sociobiology.v62i1.28-33
References based on Global Ant Biodiversity Informatics
- De Souza D. J., I. M. F. Soares, and T. M. C. Della Lucia. 2007. Acromyrmex ameliae sp. n. (Hymenoptera: Formicidae): A new social parasite of leaf-cutting ants in Brazil. Insect Science 14: 251-257.
