Lasius interjectus
| Lasius interjectus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Lasiini |
| Genus: | Lasius |
| Section: | flavus clade |
| Species group: | claviger |
| Species: | L. interjectus |
| Binomial name | |
| Lasius interjectus Mayr, 1866 | |
| Common Name | |
|---|---|
| Larger Yellow Ant | |
| Language: | English |
Primarily subterranean, this species can be found nesting under stones or logs and in the soil, or occasionally with a small mound, in a wide variety of habitats.
| At a Glance | • Temporary parasite |
Photo Gallery
Identification
The hairs of this species are long, those on the ventral surface of the head are often at least 0.20 mm in length, and those on the gaster are often at least 0.23 mm in length. Most of the hairs on the gaster are confined to the posterior edges of each terga. The apex of the petiole is moderately sharp, usually concave or notched. (Mackay and Mackay 2002)
Ellison et al., (2012) - All species in the claviger group smell like citronella when disturbed or crushed. Lasius interjectus has a sharply pointed petiole like Lasius claviger and Lasius subglaber, but L. interjectus has many long, erect hairs all over the first segment of its gaster and only on the edges of the other segments of the gaster.
Keys including this Species
- Key to Lasius-Nearctic workers of Acanthomyops short key
- Key to Lasius-Nearctic Acanthomyops workers
- Key to Lasius-Nearctic Acanthomyops queens
- Key to Lasius-Nearctic Acanthomyops males
- Key to North American Lasius Species
- Key to New England Lasius
Distribution
Lasius interjectus ranges from southern New England west to Idaho, south to Wyoming and New Mexico, and east into Oklahoma, Arkansas and Georgia.
Latitudinal Distribution Pattern
Latitudinal Range: 49.661° to 19.48833°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Biology
Wing (1968) - Of the 337 samples of this taxon, 66 had associated nest data. Nests in or adjacent to buildings numbered 27. Of the nests not associated with buildings, 10 were in the open, 16 in woods, and 13 of uncertain status. With respect to immediate nestcover, 10 were under stones, 8 associated with wood, 1 in a mound, 11 with other or no cover, and 9 of uncertain status. The kind of immediate nest-cover did not appear to be correlated with whether nests were in woods or in the open. Carter (1962b) reported on 9 nests in North Carolina. Most records were from woods or associated with buildings. Nests were in rotted stumps, under stones, and without any visible covering object in soil ranging from compact red clay to loose sand. Talbot (1963) studied 9 colonies in southern Michigan. All of these colonies were either in woods or along their edges in places where leaves had accumulated on the ground. Old, well-galleried stumps accounted for 5, 1 occupied a well-galleried log and the soil under it, and 3 were in low mounds with diameters of 3 to 4 feet. One of the mounds had been built up around a stump, which later had rotted away. Wheeler and Wheeler (1963) reported 27 samples of Lasius interjectus from North Dakota; I have seen specimens from about 1/2 of these samples. Of the 19 nests observed, they found 7 nests in grassy mounds, 7 under stones, 3 under wood, and 2 under other cover. In North Dakota nests were usually in grasslands; 14 of the 19 were found in this habitat. Of the 5 other nesting situations, 4 were at the edges of woods and 1 in woods.
Annual sexual cycle One factor complicating the interpretation of the cycle of interjectus is that it frequently infests houses. The flights from houses come to official attention far more frequently than do normal flights. The samples of this species are thus strongly biased in this respect. Most naturally occurring flights take place in June and July, with a few earlier and later. The extreme flight dates are largely determined by conditions in a given locality and year.
Taking June as the starting point of the cycle, the number of nests with alates drops in July, while the number of flying alates rises. Flights have generally stopped by September, and by October no more dealate queens can be found. The nests of interjectus may, however, still contain a few sexual individuals. The beginning of winter brings on a number of nuptial flights, usually launched into the basements of heated buildings. These unseasonable flights continue through the winter and well into the spring. The few dealate queens collected during this period arise from these flights.
Close analysis of the data indicates that a sizable proportion of the records of workers and alates for March, April, and May are probably from field nests containing adult alates. In this species, it appears that the production of alates begins in the fall or winter of the previous year. Once a nest builds up a sufficient number of alates, the existence of the right combination of environmental factors may trigger a flight. This hypothesis would account for the unusually high number of interjectus flights associated with heated buildings during the winter and early spring.
A flight that I observed in Raleigh, North Carolina, on April 20, 1949 is the earliest natural flight known to me. The nest was located in grass near a sidewalk. Large numbers of males and females were swarming out of the nest at 5:30 p.m. Males, females, and workers were intermingled, and some females were breaking away and running up and down the sidewalk. Some males were attempting to copulate with females on grass blades as the flight progressed. In general, late May is the earliest time for natural flights even in the South. This species, however, like claviger, undergoes swarm-outs. Although these formicine warm-up drills occur frequently and involve large numbers of alates, they rarely result in unseasonably early flights. The swarm-outs may, however, give rise to some "leakage" of alates, which wander too far from the nest and fail to find their way back. interjectus seems to be more strongly attracted to lights than do other species of Acanthomyops (= Lasius). Thus a number of the captures of this species in the period prior to regular flights occur at lights.
The sexual cycle of interjectus is atypical of the genus in that it nearly blankets the entire year. This is largely because the queens apparently prefer to found their colonies near buildings. The attractiveness of artificial lights may also influence their selection of nesting sites.
Talbot (1963) studied a single colony of interjectus in Livingston Co., Michigan during a 50-day period in 1960. Between June 16 and August 25, this colony, which produced only males that year, launched 22 flights. About 3 weeks of this period were unfavorable for flights; in a more favorable year the flight period might end earlier. Buren (1944) reported flights for July and early August in Iowa.
Talbot (1963) reported that June 11 was the earliest date on which she had found alates in nests. Since she normally arrived at the Reserve not much before June 11, this can only mean that some interjectus nests contained adult alates upon her arrival. Evidently adult alates occur in nests in the early spring, and perhaps in the winter.
Talbot observed 43 nests over a 10-year period; her data on this species are far more extensive than on the other 3 species studied. Rains provided the stimulus for workers to open up the nest by excavation. Flights occurred in the late afternoon, with temperatures between 67° and 80°F, relative humidity between 56 and 99 percent, and without wind. An estimate of the number of alates released during flights was made on several occasions. In 1960, when only males were produced in one colony studied, an estimated 40,000 individuals flew from the nest in 22 flights. In 1958, an estimated 63,600 males and females flew from a nest in 4 flights, and the nest still contained alates. The bulk of these alates, an estimated 55,200, were released during a 2-hour period on June 17. As flights from a given nest proceeded, fewer and fewer alates were released. In some nests alates were still left in the nest after the last flight had occurred. She noted that occasional matings took place on leaves and grass stems prior to flights.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
This species is a temporary parasite with the following known hosts: Lasius alienus, Lasius claviger, Lasius latipes, Lasius minutus, Lasius murphyi and Lasius umbratus (de la Mora et al., 2021; Raczkowski & Luque, 2011).
The report of Lasius latipes being enslaved by L. interjectus by Mackay & Mackay (2002) and Janda et al. (2004) is in conflict with Raczkowski & Luque (2011) and may involve an identification error (de la Mora et al., 2021).
Nest Guests
Wing (1968) - A pointed worker specimen had a single pointed specimen of Batrisodes montrosus (Coleoptera: Pselaphidae) associated with it. Records of insects associated with interjectus from the literature are: Schwarz (1890), Batrisodes montrosus, B. ferox, and Serica vespertina (Coleoptera: Scarabaeidae). Wickham (1900) reported on the sifting of an old stump which contained worker ants, Adranes lecontei, Ceophyllus monilis, (Coleoptera: Pselaphidae), and Limulodes paradoxus (Coleoptera: Limulodidae). The ant species was determined by Wasmann as Lasius "interjeotionis"; I believe that it is safe to assume that interjectus was the ant species associated with these guests.
Flight Period
This species is unusual in the claviger-group, and indeed in the entire genus, in that it typically flies in mid- to late spring, rather than late summer or autumn (James Trager, per. comm.).
| X | X | X | |||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
 Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Castes
Worker
Images from AntWeb
   
| |
| Worker. Specimen code casent0103545. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |

| |
| Worker. Specimen code casent0102696. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |

| |
| . | |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0103546. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
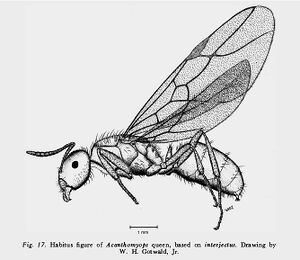
| |
| . | |
Male
Images from AntWeb
    
| |
| Male (alate). Specimen code casent0103544. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |

| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- interjectus. Lasius interjectus Mayr, 1866b: 888, pl. 20, fig. 3 (q.) U.S.A. Emery, 1893i: 642 (w.); Wing, 1968: 93 (m.); Wheeler, G.C. & Wheeler, J. 1953c: 156 (l.). Combination in Lasius (Acanthomyops): Emery, 1893i: 642; Wheeler, W.M. 1917a: 531; in Acanthomyops: Creighton, 1950a: 430; in Lasius: Ward, 2005: 13. See also: Wing, 1968: 92; Smith, D.R. 1979: 1441.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Wing (1968) - Large, HW at least 1.08 mm, most specimens measuring 1.20 mm or more; AL at least 1.28 mm, most specimens measuring 1.50 mm or more. Head wide, CI at least 98, often 100 or more. Basal margin of mandible often with 1 or more denticles. Antennae slender; SI 75 to 86, most specimens falling between 78 and 85. Propodeum convex in profile, often strongly so. Crest of petiolar scale sharp to moderately sharp, usually narrowly but distinctly emarginate; sides usually convex. Standing body hairs simple to minutely barbulate, long; those on dorsum of gaster confined to rows along posterior edges of tergites beyond first; those on gula confined to its posterior 2/3. Pubescence on dorsum of gaster dilute, that on upper part of head moderate to dense.
Moderately pilose. Pubescence on head and appendages moderate to dense, usually moderate to dense on base of gaster, elsewhere on body mostly dilute; surface of most parts of body shining. Antennal scapes with pubescence appressed to suberect. Body and appendages yellow to pale brownish yellow.
Queen
Wing (1968) - Basal margin of mandible usually with 1 or more denticles. Head broad, HW almost always 1.53 mm or more, CI 102 to 110, usually 104 or greater. Antennae long and slender, penultimate and antepenultimate segments of funiculus at least as long as wide, usually longer than wide. SL almost always over 1.08 mm, SI 64-75, usually 67 or over. Crest of petiolar scale sharp to moderately sharp, usually narrowly but distinctly emarginate; sides convex. Body size moderate, AL of most specimens falls between 2.35 and 2.85 mm. Standing body hairs finely barbulate and simple, long, fairly numerous, especially on alitrunk ; those on gula confined to its posterior 1/3 to 3/4.
Head and appendages moderately to densely pubescent, other parts of body mostly with dilute pubescence. Standing hairs on dorsum of gaster confined to posterior edges of tergites beyond first. Body color of most specimens light brownish yellow, a few sordid yellowish brown, often with gaster darker than rest of the body.
Male
Wing (1968) - Large, HW usually over 1.00 mm; AL at least 1.50 mm, usually 1.60 mm or more. Head fairly broad, CI 110 or more. Antennae slender, SL 0.70 mm or more, SI 70 or more. Crest of petiolar scale sharp to very sharp, usually distinctly emarginate; upper sides often straight and converging dorsally. Standing body hairs mostly simple, moderately numerous; many of those at posterior tip of gaster measuring over 0.27 mm; those on gula measuring 0.18 mm or more. Pubescence on dorsum of gaster dilute to very dilute, that on head and appendages moderate to dense.
Antennal scapes with pubescence ranging from appressed to suberect. Alitrunk with pubescence variable, central areas of scutum and scutellum largely free of pubescence. Color of alitrunk brown, often with a reddish cast; head and sometimes gaster darker, appendages lighter.
Type Material
Wing (1968) - Type locality: New Jersey. Location of type: Dr. Per Inge Persson, Naturhistoriska Riksmuseum, Stockholm, kindly sent me information about the type, which is in their collection. It is a queen with the following data: "N. Yersey, Belfrage." Belfrage was a Swedish collector who moved to the USA in 1850. There are no type specimens located in the collections of the Naturhistorisches Museum in Wien.
References
- Bolton, B. 1995b. A new general catalogue of the ants of the world. Cambridge, Mass.: Harvard University Press, 504 pp. (page 53, catalogue)
- Boudinot, B.E., Borowiec, M.L., Prebus, M.M. 2022. Phylogeny, evolution, and classification of the ant genus Lasius, the tribe Lasiini and the subfamily Formicinae (Hymenoptera: Formicidae). Systematic Entomology 47, 113-151 (doi:10.1111/syen.12522).
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Creighton, W. S. 1950a. The ants of North America. Bulletin of the Museum of Comparative Zoology 104: 1-585 (page 430, Combination in Acanthomyops)
- Davis, T. 2009. The ants of South Carolina (thesis, Clemson University).
- de la Mora, A., Sankovitz, M., Purcell, J. 2020. Ants (Hymenoptera: Formicidae) as host and intruder: recent advances and future directions in the study of exploitative strategies. Myrmecological News 30: 53-71 (doi:10.25849/MYRMECOL.NEWS_030:053).
- Ellison, A.M., Gotelli, N.J., Farnsworht, E.J., Alpert, G.D. 2012. A Field Guide to the Ants of New England. Yale University Press, 256 pp.
- Emery, C. 1893k. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 7: 633-682 (page 642, Combination in Lasius (Acanthomyops))
- Emery, C. 1893k. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 7: 633-682 (page 642, worker described)
- Ipser, R.M., Brinkman, M.A., Gardner, W.A., Peeler, H.B. 2004. A survey of ground-dwelling ants (Hymenoptera: Formicidae) in Georgia. Florida Entomologist 87: 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87 (doi:10.3897@jhr.70.35207).
- MacGown, J.A., Booher, D., Richter, H., Wetterer, J.K., Hill, J.G. 2021. An updated list of ants of Alabama (Hymenoptera: Formicidae) with new state records. Transactions of the American Entomological Society 147: 961-981 (doi:10.3157/061.147.0409).
- Mackay, W. P. and E. Mackay. 2002. The ants of New Mexico (Hymenoptera: Formicidae). Edwin Mellen Press, Lewiston, NY.
- Mayr, G. 1866b. Diagnosen neuer und wenig gekannter Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 16: 885-908 (page 888, pl. 20, fig. 3 queen described)
- Raczkowski, J.M. & Luque, G.M. 2011. Colony founding and social parasitism in Lasius (Acanthomyops). Insectes Sociaux 58: 237‐244.
- Rericha, L. 2007. Ants of Indiana. Indiana Department of Natural Resources, 51pp.
- Smith, D. R. 1979. Superfamily Formicoidea. Pp. 1323-1467 in: Krombein, K. V., Hurd, P. D., Smith, D. R., Burks, B. D. (eds.) Catalog of Hymenoptera in America north of Mexico. Volume 2. Apocrita (Aculeata). Washington, D.C.: Smithsonian Institution Press, pp. i-xvi, 1199-2209. (page 1441, see also)
- Ward, P.S. 2005. A synoptic review of the ants of California (Hymenoptera: Formicidae). Zootaxa 936: 1-68 (page 13, revived combination in Lasius (Acanthomyops))
- Waters, J.S., Keough, N.W., Burt, J., Eckel, J.D., Hutchinson, T., Ewanchuk, J., Rock, M., Markert, J.A., Axen, H.J., Gregg, D. 2022. Survey of ants (Hymenoptera, Formicidae) in the city of Providence (Rhode Island, United States) and a new northern-most record for Brachyponera chinensis (Emery, 1895). Check List 18(6), 1347–1368 (doi:10.15560/18.6.1347).
- Wheeler, G. C.; Wheeler, J. 1953c. The ant larvae of the subfamily Formicinae. Ann. Entomol. Soc. Am. 46: 126-171 (page 156, larva described)
- Wheeler, W. M. 1917a. The mountain ants of western North America. Proc. Am. Acad. Arts Sci. 52: 457-569 (page 531, Combination in Lasius (Acanthomyops))
- Wing, M. W. 1968a. Taxonomic revision of the Nearctic genus Acanthomyops (Hymenoptera: Formicidae). Mem. Cornell Univ. Agric. Exp. Stn. 405: 1-173 (page 92, see also)
- Wing, M. W. 1968a. Taxonomic revision of the Nearctic genus Acanthomyops (Hymenoptera: Formicidae). Mem. Cornell Univ. Agric. Exp. Stn. 405: 1-173 (page 93, male described)
References based on Global Ant Biodiversity Informatics
- Allred D. M. 1982. Ants of Utah. The Great Basin Naturalist 42: 415-511.
- Allred, D.M. 1982. The ants of Utah. Great Basin Naturalist 42:415-511.
- Carroll T. M. 2011. The ants of Indiana (Hymenoptera: Formicidae). Master's Thesis Purdue university, 385 pages.
- Clark A. T., J. J. Rykken, and B. D. Farrell. 2011. The Effects of Biogeography on Ant Diversity and Activity on the Boston Harbor Islands, Massachusetts, U.S.A. PloS One 6(11): 1-13.
- Cokendolpher J. C., and O. F. Francke. 1990. The ants (Hymenoptera, Formicidae) of western Texas. Part II. Subfamilies Ecitoninae, Ponerinae, Pseudomyrmecinae, Dolichoderinae, and Formicinae. Special Publications, the Museum. Texas Tech University 30:1-76.
- Cole A. C. 1953. A checklist of the ants (Hymenoptera: Formicidae) of the Great Smoky Mountains National Park, Tennessee. Journal of the Tennessee Academy of Science. 28: 34-35.
- Cole A. C., Jr. 1942. The ants of Utah. American Midland Naturalist 28: 358-388.
- Cole A. C., Jr. 1954. Studies of New Mexico ants. XIII. The genera Acanthomyops, Myrmecocystus, and Polyergus (Hymenoptera: Formicidae). Journal of the Tennessee Academy of Science 29: 284-285.
- Coovert G. A. 2005. The Ants of Ohio (Hymenoptera: Formicidae). Ohio Biological Survey, Inc. 15(2): 1-207.
- Coovert, G.A. 2005. The Ants of Ohio (Hymenoptera: Formicidae) Ohio Biological Survey Bulletin New Series Volume 15(2):1-196
- Del Toro, I. 2010. PERSONAL COMMUNICATION. MUSEUM RECORDS COLLATED BY ISRAEL DEL TORO
- Deyrup M. 2016. Ants of Florida: identification and natural history. CRC Press, 423 pages.
- Deyrup, M. 2003. An updated list of Florida ants (Hymenoptera: Formicidae). Florida Entomologist 86(1):43-48.
- Dubois, M.B. and W.E. Laberge. 1988. An Annotated list of the ants of Illionois. pages 133-156 in Advances in Myrmecology, J. Trager
- Ellison A. M., S. Record, A. Arguello, and N. J. Gotelli. 2007. Rapid Inventory of the Ant Assemblage in a Temperate Hardwood Forest: Species Composition and Assessment of Sampling Methods. Environ. Entomol. 36(4): 766-775.
- Ellison A. M., and E. J. Farnsworth. 2014. Targeted sampling increases knowledge and improves estimates of ant species richness in Rhode Island. Northeastern Naturalist 21(1): NENHC-13NENHC-24.
- Emery C. 1893. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 7: 633-682.
- General D.M. & Thompson L.C. 2007. Ants (Hymenoptera: Formicidae) of Arkansas Post National Memorial. Journal of the Arkansas Acaedemy of Science. 61: 59-64
- Guénard B., K. A. Mccaffrey, A. Lucky, and R. R. Dunn. 2012. Ants of North Carolina: an updated list (Hymenoptera: Formicidae). Zootaxa 3552: 1-36.
- Ipser R. M. 2004. Native and exotic ants (Hymenoptera: Formicidae) of Georgia: Ecological Relationships with implications for development of biologically-based management strategies. Doctor of Philosophy thesis, University of Georgia. 165 pages.
- Ipser, R.M., M.A. Brinkman, W.A. Gardner and H.B. Peeler. 2004. A Survey of Ground-Dwelling Ants (Hymenoptera: Formicidae) in Georgia. The Florida Entomologist 87(3) 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87.
- Ivanov K., L. Hightower, S. T. Dash, and J. B. Keiper. 2019. 150 years in the making: first comprehensive list of the ants (Hymenoptera: Formicidae) of Virginia, USA. Zootaxa 4554 (2): 532–560.
- Johnson R. Personnal Database. Accessed on February 5th 2014 at http://www.asu.edu/clas/sirgtools/resources.htm
- Lubertazi, D. Personal Communication. Specimen Data from Museum of Comparative Zoology at Harvard
- Lynch J. F. 1988. An annotated checklist and key to the species of ants (Hymenoptera: Formicidae) of the Chesapeake Bay region. The Maryland Naturalist 31: 61-106
- Mackay W. P., and E. E. Mackay. 2002. The ants of New Mexico (Hymenoptera: Formicidae). Lewiston, New York: Edwin Mellen Press, 400 pp.
- Mackay, W., D. Lowrie, A. Fisher, E. Mackay, F. Barnes and D. Lowrie. 1988. The ants of Los Alamos County, New Mexico (Hymenoptera: Formicidae). pages 79-131 in J.C. Trager, editor, Advances in Myrmecololgy.
- Merle W. W. 1939. An Annotated List of the Ants of Maine (Hymenoptera: Formicidae). Entomological News. 50: 161-165
- Nuhn, T.P. and C.G. Wright. 1979. An Ecological Survey of Ants (Hymenoptera: Formicidae) in a Landscaped Suburban Habitat. American Midland Naturalist 102(2):353-362
- O'Keefe S. T., J. L. Cook, T. Dudek, D. F. Wunneburger, M. D. Guzman, R. N. Coulson, and S. B. Vinson. 2000. The Distribution of Texas Ants. The Southwestern Entomologist 22: 1-92.
- Ouellette G. D., F. A. Drummond, B. Choate and E. Groden. 2010. Ant diversity and distribution in Acadia National Park, Maine. Environmental Entomology 39: 1447-1556
- Procter W. 1938. Biological survey of the Mount Desert Region. Part VI. The insect fauna. Philadelphia: Wistar Institute of Anatomy and Biology, 496 pp.
- Roeder K. A., and D. V. Roeder. 2016. A checklist and assemblage comparison of ants (Hymenoptera: Formicidae) from the Wichita Mountains Wildlife Refuge in Oklahoma. Check List 12(4): 1935.
- Smith F. 1941. A list of the ants of Washington State. The Pan-Pacific Entomologist 17(1): 23-28.
- Smith M. R. 1934. A list of the ants of South Carolina. Journal of the New York Entomological Society 42: 353-361.
- Smith M. R. 1935. A list of the ants of Oklahoma (Hymen.: Formicidae) (continued from page 241). Entomological News 46: 261-264.
- Smith M. R. 1952. On the collection of ants made by Titus Ulke in the Black Hills of South Dakota in the early nineties. Journal of the New York Entomological Society 60: 55-63.
- Talbot M. 1976. A list of the ants (Hymenoptera: Formicidae) of the Edwin S. George Reserve, Livingston County, Michigan. Great Lakes Entomologist 8: 245-246.
- Warren, L.O. and E.P. Rouse. 1969. The Ants of Arkansas. Bulletin of the Agricultural Experiment Station 742:1-67
- Wheeler G. C., J. N. Wheeler, and P. B. Kannowski. 1994. Checklist of the ants of Michigan (Hymenoptera: Formicidae). The Great Lakes Entomologist 26(4): 297-310
- Wheeler G. C., and E. W. Wheeler. 1944. Ants of North Dakota. North Dakota Historical Quarterly 11:231-271.
- Wheeler G. C., and J. Wheeler J. 1989. A checklist of the ants of Oklahoma. Prairie Naturalist 21: 203-210.
- Wheeler G. C., and J. Wheeler. 1987. A Checklist of the Ants of South Dakota. Prairie Nat. 19(3): 199-208.
- Wheeler W. M. 1905. An annotated list of the ants of New Jersey. Bulletin of the American Museum of Natural History. 21: 371-403.
- Wheeler W. M. 1906. Fauna of New England. 7. List of the Formicidae. Occasional Papers of the Boston Society of Natural History 7: 1-24.
- Wheeler, G.C. and J. Wheeler. 1988. A checklist of the ants of Montana. Psyche 95:101-114
- Wheeler, G.C. and J. Wheeler. 1988. A checklist of the ants of Wyoming. Insecta Mundi 2(3&4):230-239
- Wheeler, G.C., J. Wheeler and P.B. Kannowski. 1994. CHECKLIST OF THE ANTS OF MICHIGAN (HYMENOPTERA: FORMICIDAE). Great Lakes Entomologist 26:1:297-310
- Wing M. W. 1939. An annotated list of the ants of Maine (Hymenoptera: Formicidae). Entomological News 50:161-165.
- Wing M. W. 1968. Taxonomic revision of the Nearctic genus Acanthomyops (Hymenoptera: Formicidae). Memoirs of the Cornell University Agricultural Experiment Station 405: 1-173.
- Young J., and D. E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publication. Oklahoma Agricultural Experimental Station 71: 1-42.
- Young, J. and D.E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publications of Oklahoma State University MP-71
- Pages using DynamicPageList3 parser function
- Common Name
- Temporary parasite
- Photo Gallery
- North temperate
- North subtropical
- Tropical
- Ant Associate
- Host of Lasius alienus
- Host of Lasius claviger
- Host of Lasius latipes
- Host of Lasius minutus
- Host of Lasius murphyi
- Host of Lasius umbratus
- FlightMonth
- Species
- Extant species
- Formicidae
- Formicinae
- Lasiini
- Lasius
- Lasius interjectus
- Formicinae species
- Lasiini species
- Lasius species
- Ssr




