Strumigenys lewisi
| Strumigenys lewisi | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Strumigenys |
| Species: | S. lewisi |
| Binomial name | |
| Strumigenys lewisi Cameron, 1886 | |
| Common Name | |
|---|---|
| Uroko-ari | |
| Language: | Japanese |
Nests are found under stones or logs, in the soil, decayed stumps or bamboo stems. Winged forms appear in August. Details of predatory behavior were reported by Masuko (1984, 1985) (see Hunting Tactics in Short-Mandibulate Strumigenys for details). The workers prey on Collembola (Okamoto, 1953, Masuko, 1984). Colonies are small, usually with between tens and 100 workers (Teranishi, 1929). The species is polygynous. It is one of the most common soil-inhabiting ants of Japan. (Japanese Ant Image Database) Collects are known in both closed and open broadleaf forest, from elevations up to 1180 m (Tang & Guenard, 2023).
| At a Glance | • Polygynous • Limited invasive |
Identification
Bolton (2000) - A member of the godeffroyi complex in the Strumigenys godeffroyi-group. See notes under Strumigenys godeffroyi.
Yoshimura and Onoyama (2007) - Strumigenys lewisi and Strumigenys kumadori are very similar to Strumigenys geminata. S. lewisi and S. kumadori can be separated from the latter by having a more feeble propodeal spine and the lateral surface of pronotum completely reticulate-punctate or at most with a small smooth patch above the fore coxa.
Strumigenys kumadori is distinguished from S. lewisi by the following characters: 1) in the workers, all of two paired hairs on the apicoscrobe and anterior portion of the mesonotum are long and distinctly flagellate in the former, but both of the hairs are plumose-filiform or filiform in the latter (Figs 12,13 – see figures in caste section below and in the caste section for Strumigenys kumadori), 2) the eyes of the queens are relatively large in the former, but relatively small in the latter (Fig. 56), 3) the lateral ocelli of queens are distinctly large (Figs 20, 55) with pigmented outer margins in the former, but relatively small (Figs 26, 55) or vestigial and not distinctly bordered with pigment around them in the latter, 4) the mesoscutum of queen in dorsal view is wide (MsWI>63) and weakly constricted at posterior 1/3, and lateral corners by the constriction are not angular in the former, but narrow (MsWI<63) and strongly constricted, and the lateral corners are angular in the latter, 5) the transverse furrow on mesoscutum of queen is curved posteriorly in the former (Fig. 19), but nearly straight in the latter (Fig. 25), 6) fore wings of queen are relatively broad in the former (Fig. 41), but are reduced and narrow in the latter (Fig. 43), 7) the radial vein on the hind wing does not reach the costal margin in the former (Figs 41, 42), but does so in the latter (Figs 43, 44), 8) the mandible of male is relatively narrow in the former (Figs 30, 32), but is relatively wide in the latter (Figs 36, 38), 9) on the male genitalia in lateral view, volsella is gently curved and the corner is not broadened in the former (Fig. 46), but is abruptly curved and the corner is distinctly broadened in the latter (Fig. 50).
In the present study, the characters in the queen have most distinctly separated between the two species, S. kumadori and S. lewisi. The eyes, ocelli, mesonotum, and wings are useful characters to distinguish these two species. While much developed, the male's mesosoma did not provide useful characters to separate the two species, while the queen's one did. The reduced male mandible, however, provided useful distinguishing characters.
Keys including this Species
Distribution
Bolton (2000) - Bingham (1903) recorded this species from Burma but the record has not been confirmed in this survey and is probably incorrect; the specimens, now lost, may perhaps have been Strumigenys peraucta, a newly described Indian species of this complex. W. M. Wheeler (1935) and Wilson & Taylor (1967) record lewisi from Hawaii, where it is an introduction, and Arakelian & Dlussky (1991) record it on Kunashir Island in the Kuril'skiye Ostrova, which is almost certainly part of its normal range.
Tang & Guenard (2023) - One of the most widespread and well-documented species of Strumigenys in Asia, with its geographical range including central to northern provinces of mainland China (as far as Beijing) and North Korea, which are often void of any record of Strumigenys. Its presence in Chinese provinces such as Hainan, Hebei, Henan, Anhui, Jiangxi is likely. Records from Myanmar need confirmation (Bolton 2000), while past records from India have been excluded (Bharti et al. 2016). Similarly, several records of this species outside its native range such as in Malta and Georgia in Europe most likely represent misidentifications (Hamer et al. 2021). Records from the Philippines (Way et al. 1998) would also need to be confirmed.
Latitudinal Distribution Pattern
Latitudinal Range: 38.283333° to 29.54°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Indo-Australian Region: Niue, Philippines.
Oriental Region: Myanmar, Taiwan, Vietnam.
Palaearctic Region: China, Democratic Peoples Republic of Korea, Japan (type locality), Malta, Republic of Korea.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Yoshimura and Onoyama (2007) - Our collection data of the colonies suggest that polygyny is common in S. lewisi and Masuko et al. (1985) reported S. lewisi is predominantly polygynous.
Latitudinal variations of S. lewisi: Two morphological indices, DlOI and MsHI, showed negative tendencies with latitudinal gradient. The relationship between DlOI and latitude (r=-0.614, n=34, P<0.0001) was significant, although that between MsHI and latitude was not significant (r=-0.314, n=34, P=0.071). Reduction of the mesoscutum, ocelli, and wings suggest that the ability to fly is degenerated. The degeneration in the queens gives us two hypotheses for S. lewisi; 1) a range of the dispersion in northern population is smaller than that in southern population, 2) the northern population has dispersed their genes relying on the flying ability of the males.
The distribution areas and sites of S. kumadori and S. lewisi. overlap in Honshu, Kyushu, and Korea (JADG, 2003a, 2003b, 2003c; Terayama, 1999). We additionally confirm the presence of both species in Taiwan. Nest habitats of the two species are the same or very similar (Masuko et al., 1985), and we actually confirmed that both species were collected from a single leaf litter sample taken from a quadrat of 0.5m x 0.5m. Therefore, these two have completely sympatric distribution.
Brown (1949) - The type of lewisi came from Japan, and the species is apparently common in the warm temperate parts of Japan, China and adjacent islands, including the Ryukyus. It has been reported also from Hawaii and Upper Burma, and Dr. M. R. Smith has sent me specimens taken at U. S. Plant Quarantine Stations in ginger root; it is also known to have reached other points on the shores of Pacific in shipments of timber and in the earth about living plants. Mr. F. G. Werner has turned over to me specimens taken by Dr. C. T. Parsons at Kanna, Okinawa, and I have taken several nests numbering 200 to 250 workers each on the densely populated irrigated plains around Chengtu, Szechuan Province. One of these nests (Chengtu) was intermingled in a colony of a small yellow Tetramorium under a small board; the other (Hsuangliu) accompanying a different species was with a common blackish Aphaenogaster nesting in the soil of a farm compound among extensive rice paddies. Up to six queens were found in colonies of lewisi examined.
Flight Period
| X | |||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Notes: Japan.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
 Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Life History Traits
- Queen number: polygynous
- Mean colony size: 70 (Teranishi, 1929) (reported as "between tens and 100")
- Maximum colony size: 100 (Teranishi, 1929) (reported as "between tens and 100")
Castes
Worker
  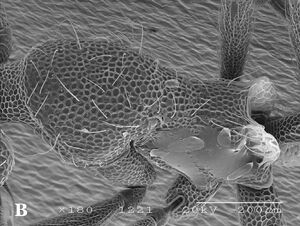     
| |
| . | |
Queen

| |
| . | |
Male

| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- lewisi. Strumigenys lewisi Cameron, 1886: 229 (w.q.) JAPAN. Wheeler, G.C. & Wheeler, J. 1955a: 140 (l.). Subspecies of godeffroyi: Mayr, 1887: 569 (footnote); Wheeler, W.M. 1906c: 318; Emery, 1924d: 321; Wheeler, W.M. 1923b: 4; Wheeler, W.M. 1928d: 115. Revived status as species: Bingham, 1903: 149; Brown, 1949d: 16. See also: Bolton, 2000: 794; Yoshimura & Onoyama, 2007: 671.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Bolton (2000) - TL 2.6-2.7, HL 0.65-0.70, HW 0.45-0.48, CI 68-71, ML 0.30-0.32, MI 45-47, SL 0.38-0.42, SI 83-89, PW 0.28-0.30, AL 0.70-0.73 (10 measured).
Characters of godeffroyi complex. Cephalic dorsum with pair of erect hairs closest to midline on occipital margin short stiff and erect, straight to shallowly evenly curved but the apical half not abruptly curved anteriorly nor looped. With head in full-face view the dorsolateral margin posterior to the flagellate apicoscrobal hair has a row of 3-4 stiffly projecting hairs. These hairs contrast with the marginal hairs anterior to the flagellate hair as they are more cylindrical (i.e. not spatulate), more elevated and less strongly curved anteriorly. Ground-pilosity on pronotal dorsum sparse and dilute, not appearing as a pelt. Dorsum and side of pronotum evenly finely reticulate-punctate everywhere or with a small smooth patch on the side above the fore coxa. Dorsum of pronotum with a pair of erect hairs in addition to the humeral pair. In most samples this pair of hairs is flagellate but in some the hairs are looped apically and in some they appear filiform, though these may represent breakage. Pair of erect hairs on mesonotum usually flagellate but again in some individuals they may be looped or appear filiform. Pleurae and side of propodeum mostly to entirely smooth, any reticulate-punctate sculpture present is confined to periphery. Propodeal declivity with a broad and very conspicuous lamella, the propodeal teeth at most only weakly expressed (may be vestigial or indistinguishable from the lamella), entirely confluent with the lamella. Disc of postpetiole unsculptured. Basigastral costulae conspicuous but not extending half the length of the tergite.
Yoshimura and Onoyama (2007) - HL 0.65 - 0.71, HW 0.48 - 0.50 CI 69.8 - 76.0, ML 0.31 - 0.35, MI 46.9 - 50.0, SL 0.37 - 0.40, SI 76.4 - 82.0, DSA 3 L 0.12 - 0.13, DSA 3 W 0.19 - 0.20, DSA 3 I 140.0 - 155.6 (6 measured).
Ventrolateral margin of head at level of eye not extended outward. Antenna consisting of 6 segments. Fully closed mandible in full-face view curvilinear. On the mandible, a distinct, long and spiniform preapical tooth present close to apical teeth. Apical teeth consisting of two distinct spiniform teeth and three small intercalary teeth between them: basal of the two spiniform teeth longer than the apical one: basal one of the three intercalary teeth distinctly smaller than apical two. With mesosoma in lateral view, the diameter of the excavated area of mesopleural gland moderate, much less than the maximum width of the first coxa. Mesosoma except for propodeal declivity without spongiform tissue. Propodeal declivity equipped with a broad and conspicuous lamella; propodeal tooth very feeble and not sclerotized; posterior margin of the lamella convex, and immediately under the propodeal tooth of the margin sometimes slightly concave. Ventral margin of petiole in lateral view with longitudinal spongiform tissue. With petiole in lateral view, anteriormost point of lateral spongiform lobe nearly reaching level of anterior face of node.
Dorsal and lateral surfaces of pronotum entirely reticulate-punctate, sometimes with a small patch above the fore coxa. Metapleuron and side of propodeum entirely smooth. Limbus distinct. Abdominal tergite IV longitudinally sculptured at the basal portion, but not entirely covered.
With head in full-face view, a pair of long, curved distally plumose filiform hairs, rarely not serrate, present on apicoscrobe; hairs posterior to the apicoscrobal hairs with shorter barbs, laterally projecting filiform hairs; anterior to the apicoscrobal hairs without laterally projecting hair. With head in lateral view, dorsal surface from level of eye to preoccipital margin with erect to reclinate ground-pilosity; hair on vertex margin distinctly differentiated from that on level of eye; from highest point of vertex to preoccipital margin with the anteriorly directed ground-pilosity, which is very feebly curved basally so that each hair is elevated and inclined upward away from the cephalic outline. A pair of hairs present on the pronotal humeri and mesonotum; those on the humeri usually flagellate; those on the mesonotum usually short filiform hairs but sometimes long curved. Dorsum of hind femur without short erect hairs, but with two or three (usually two) long erect flagellate hairs. Dorsal surface of hind basitarsus with one freely projecting flagellate hair. The whole of the dorsal surface of abdominal tergite IV with flagellate hairs. Basal portion of abdominal sternite IV covered with matted hair-like tissue.
Body almost unicolorous, reddish brown to yellowish brown.
Queen
Yoshimura and Onoyama (2007) - HL 0.63 - 0.70, HW 0.45 - 0.52 CI 71.5 - 80.0, ML 0.29 - 0.33, MI 44.8 - 49.2, SL 0.33 - 0.38, SI 67.9 - 76.2, DlO 0 - 0.02, DlOI 0 - 4.94, EL 0.08 - 0.11, EI 16.6 - 21.6, HD 0.30 - 0.32, PrH 0.18 - 0.22, MsW 0.26 - 0.31, MsWI 50.4 - 60.6, MsH 0.07 - 0.13, MsHI 14.2 - 27.0, DSA 3 L 0.12 - 0.15, DSA 3 W 0.19 - 0.24, DSA 3 I 146.9 - 188.7 (10 measured).
Generally similar to the worker with the usual caste differences. Head thinner than that of queen of Strumigenys kumadori in lateral view. With head in full-face view, the ocelli weakly developed situated at posterior 1 / 4 of the head with brown pigment around them, but ocelli often vestigial and visible only with the pigments. Eye relatively small. A distinct, long spiniform preapical tooth present close to apical teeth. Apical teeth consisting of two spiniform teeth and three, rarely two, small intercalary teeth: basal one of the two spiniform teeth longer than another apical one: basal one of the three (or two) intercalary teeth distinctly smaller than the apical two (or the apical one). With mesosoma in lateral view, the highest point of the mesoscutum situated anterior to extension line of the mesopleural wing process in most cases; mesopleural gland orifice distinct but its maximum width not reaching maximum width of the procoxa; the pits on the mesepisternum invisible. Metanotum in lateral view slightly convex posteriorly. Propodeal spine developed and weakly sclerotized, and under which the lobe of spongiform tissue distinctly developed. With the spongiform tissue on propodeal declivity in lateral view, its posterior margin weakly concave under the propodeal spine. With mesoscutum in dorsal view, its anterior margin relatively sharp, both lateral margins strongly constricted at posterior 1 / 3, lateral corners by the constriction strongly angular. Transverse furrow on the mesoscutum nearly straight. Mesoscutum narrow (MsWI less than 63), its width not reaching 3 / 4 of the head width in frontal view. With petiole in lateral view, the lobe of spongiform tissue strongly developed.
Both of the fore and hind wings distinctly reduced in width at distal 1 / 2. Only costal (C) and radial (R 1) veins and r-rs cross vein clearly present on fore wing. Vestiges of the radial sector (Rs), M + Cu, and cubital (Cu) veins sometimes visible as pigmented lines but not sclerotized. On the hind wing, radial (R) vein present, reaching to costal margin and extended distally; jugal lobe absent.
Head, pronotum, mesonotum, and metanotum entirely reticulate-punctate. Central part of mesepisternum and most part of propodeum ventral to propodeal spiracle not punctate and smooth. Dorsal margin of petiole reticulate-punctate. Dorsal surface of postpetiole not punctate and smooth. Limbus present on abdominal tergite IV. Abdominal tergite IV longitudinally sculptured at the basal portion, but sculptures not extended to posterior half of the tergite.
Hairs on the pronotal humeri long and flagellate. Mesonotal dorsum with erect, straight or flagellate hairs. Dorsum of hind femur without short erect hairs, but with 2 or 3 (usually 2) long erect flagellate hairs. Dorsal surface of abdominal tergite IV with long filiform hairs. Hair-like tissue on the basal portion of abdominal sternite IV developed. Fore and hind wings densely hairy.
Body almost unicolorous, reddish brown to yellowish brown
Male
Yoshimura and Onoyama (2007) - HL 0.44 - 0.46, HW 0.43 - 0.46, CI 95.9 - 101.3, SL 0.09 - 0.11, SI 20.8 - 23.0, DlO 0.05 - 0.06, EL 0.18 - 0.19, HD 0.36 - 0.37, PrH 0.21 - 0.22, MsW 0.40, MsWI 91.5 - 94.0, MsH 0.16 - 0.17, MsHI 35.1 - 38.7 (3 measured).
With head in full-face view, portion of posterior to the eyes subglobose; anterior to the eyes distinctly narrowed anteriorly. Ocelli distinct; the median ocellus situated about posterior 1 / 4 of the head length, the lateral ocelli not reaching to the posterior border of the head. Eyes distinctly developed and prominent, occupying central 1 / 3 of lateral margin of the head in full-face view. Eye in lateral view broadened ventrally, and its outer margin expanded anteroventrally and flattened posteriorly. Anterior tentorial pits unclear. Anterior margin of the clypeus in full-face view slightly convex, but nearly straight. Frontal carinae undeveloped and antennal insertions exposed. Antennae long and filiform, consisting 13 segments. Scape short and broad. Pedicel short and broadened apically. With mandible in full-face view, its apical portion gradually curved and narrowed; the basal lamella distinctly recognized and strongly projected; apical to the lamella edentate. Mandible in lateral view subtriangular, but broader than that of Strumigenys kumadori . With labrum in full-face view; its apical portion distinctly extended laterally; the distal lobes entirely reduced, and apical margin of the labrum concave toward the midpoint. Palp formula 1, 1 (1 observed on SEM). Mesosoma in lateral view shorter and higher than that of the queen. Mesoscutum distinctly developed and strongly raised dorsally in lateral view. Mesoscutellum developed and slightly extended posteriorly. With the mesonotum in dorsal view, the median notal suture weakly impressed but mostly invisible; the notauli weakly impressed; the parapsidal furrows impressed and continued to the distinct transscutal suture; anterior margin of the scuto-scutellar suture distinctly sculptured longitudinally, but weak on the lateral portion, so that the division of the axillae often indistinct. Metanotum in lateral view slightly extended posteriorly. With the propodeum in lateral view, a distinct spiracle situated at the midheight; the posterior margin with distinct corner, but the spine or dent reduced; the lamella absent ventral to the propodeal corner, even if its ventral portion with a carina along the propodeal declivity. With the petiole in lateral view, the node more gently raised than that of worker and queen; the lateral spongiform lobe entirely reduced; the longitudinal spongiform tissue feebly present. Ventral margin of abdominal sternite III without a distinct process except for its extreme anterior part. Abdominal segment IV in lateral view thicker than that of worker and queen, the ventral expansion more gentle.
With genitalia in ventral view, the basal ring broader than long; lateral margins of the parameral plate weakly concave; the cuspis of volsella distinctly shorter than the digitus. With genitalia in lateral view, an anteriorly directing process, such as the barb, present at apical 1 / 4 of its ventral margin; the digitus of volsella abruptly curved ventrally and broadened at the corner.
Only costal (C) and radial (R 1) veins and r-rs cross vain clearly present on fore wing. Vestiges of the radial sector (Rs), M + Cu, and cubital (Cu) veins sometimes visible as pigmented lines but not sclerotized. On the hind wing, radial (R) vein present, reaching costal margin and extended distally; jugal lobe absent.
Head, pronotum, mesonotum, and metanotum entirely reticulate-punctate. Central part of mesepisternum and most part of propodeum ventral to propodeal spiracle not punctate and smooth. Dorsal margin of petiole reticulate-punctate. Dorsal surface of postpetiole not punctate and smooth. Limbus absent.
Two pairs of standing filiform hairs present on vertex. With head in lateral view, long and frontally projecting hairs absent anterior to median ocellus. Mesonotum with long, erect, and filiform to flagellar hairs present. Dorsal surface of the petiole, abdominal tergite III and IV with sparse filiform hairs.
Body almost unicolorous, blackish brown to reddish brown, legs same or lighter.
Type Material
Syntype workers and queen, JAPAN: Nagasaki, 3.iii. (G. Lewis) (The Natural History Museum) [examined].
References
- Baltazar, C.R. 1966. A catalogue of Philippine Hymenoptera (with a bibliography, 1758-1963). Pacific Insects Monographs 8: 1-488. (page 252, listed)
- Bingham, C. T. 1903. The fauna of British India, including Ceylon and Burma. Hymenoptera, Vol. II. Ants and Cuckoo-wasps. London: Taylor and Francis, 506 pp. (page 149, revived status as species)
- Bolton, B. 2000. The ant tribe Dacetini. Memoirs of the American Entomological Institute. 65:1-1028. (page 794, redescription of worker)
- Borowiec, L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Brown, W. L., Jr. 1949f. Revision of the ant tribe Dacetini. I. Fauna of Japan, China and Taiwan. Mushi. 20:1-25. (page 16, revived status as species)
- Cameron, P. 1886. On a new species of Strumigenys (S. lewisi) from Japan. Proc. Manch. Lit. Philos. Soc. 25: 229-232 (page 229, worker, queen described)
- Emery, C. 1924f [1922]. Hymenoptera. Fam. Formicidae. Subfam. Myrmicinae. [concl.]. Genera Insectorum 174C: 207-397 (page 321, subspecies/variety of godeffroyi)
- Hisasue, Y. 2018. Ant fauna of Matsuyama Castle. ARI 39: 18-36.
- Hisasue, Y. 2020. A checklist of the ants of Mt. Hiko-san (Kyushu, Japan). Korasana 93: 31-38.
- Imai, H.T., Kihara, A., Kondoh, M., Kubota, M., Kuribayashi, S., Ogata, K., Onoyama, K., Taylor, R.W., Terayama, M., Yoshimura, M., Ugawa, Y. 2003. Ants of Japan. 224 pp, Gakken, Japan.
- Kim, G., Lyu, D. 2012. Distribution of Ants (Insecta, Hymenoptera) in Chiaksan Mountain, Prov. Gangweon, Korea. Journal of Korean Nature 5, 127–129 (doi:10.7229/jkn.2012.5.2.127).
- Kwon, T.-S. 2015. Ant assemblages along the Baekdudaegan Mountain Range in South Korea: Human roads and temperature niche. Journal of Asia-Pacific Biodiversity 8, 152–157 (doi:10.1016/j.japb.2015.05.001).
- Lyu, Dongpyeo. 2007. A new species of Strumigenys (Hymenoptera: Formicidae) from Korea. Journal of Asia-Pacific Entomology. 10(2):117-120.
- Masuko, K. 1984. Studies on the predatory biology of oriental dacetine ants (Hymenoptera: Formicidae). I. Some Japanese species of Strumigenys, Pentastruma, and Epitritus, and a Malaysian Labidogenys, with special reference to hunting tactics in short-mandibulate forms. Insectes Sociaux. 31(4):429-451. doi:10.1007/BF02223658
- Mayr, G. 1887. Südamerikanische Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 37: 511-632 (page 569, subspecies/variety of godeffroyi)
- Michlewicz, M. 2022. Strumigenys emmae (Emery, 1890) (Hymenoptera: Formicidae) in Poland – first record of this pantropic ant species from Europe with remarks on its biology. Annals of the Upper Silesian Museum in Bytom, Entomology 31 (online 007): 1-5 (doi:10.5281/ZENODO.6559247).
- Park, S.-H., Hosoishi, S., Ogata, K., Kasuya, E. 2014. Changes of species diversity of ants over time: A case study in two urban parks. Journal of the Faculty of Agriculture, Kyushu University 59(1), 71–76.
- Park, S.-H., Hosoishi, S., Ogata, K., Kuboki, Y. 2014. Clustering of ant communities and indicator species analysis using self-organizing maps. Comptes Rendus Biologies 337, 545–552 (doi:10.1016/j.crvi.2014.07.003).
- Purkaret, A., Repta, F., Selnekovic, D., Jancik, L., Holecova, M. 2021. Notes on Strumigenys argiola (Emery, 1869) (Hymenoptera: Formicidae) with emphasis on its distribution, ecology and behaviour. Entomofauna Carpathica 33(2): 73-88.
- Ryu, J., Kim, Y.-K., Suh, S.J., Choi, K.S. 2021. The Insect database in Dokdo, Korea: An updated version in 2020. Biodiversity Data Journal 9, e62011 (doi:10.3897/bdj.9.e62011).
- Saito-Morooka, F., Fukuhara, K., Suda, K. 2015. The ant fauna of Rissho University at Kumagaya, Saitama Prefecture (Insecta, Hymenoptera, Formicidae). Bulletin of geo-environmental science (17), 35-39.
- Tang, K. L., Guénard, B. 2023. Further additions to the knowledge of Strumigenys (Formicidae: Myrmicinae) within South East Asia, with the descriptions of 20 new species. European Journal of Taxonomy 907, 1–144 (doi:10.5852/ejt.2023.907.2327).
- Wang, C., Lin, C.-C., Keller, R.A., Billen, J. 2021. The ‘hairwheels’ in Strumigenys ants are not glandular. Asian Myrmecology 13: e013004 (doi:10.20362/am.013004).
- Wheeler, G. C.; Wheeler, J. 1955a [1954]. The ant larvae of the myrmicine tribes Basicerotini and Dacetini. Psyche (Camb.) 61: 111-145 (page 140, larva described)
- Wheeler, W. M. 1906h. The ants of Japan. Bull. Am. Mus. Nat. Hist. 22: 301-328 (page 318, subspecies/variety of godeffroyi)
- Wheeler, W. M. 1923c. Chinese ants collected by Professor S. F. Light and Professor A. P. Jacot. American Museum Novitates 69: 1-6 (page 4, subspecies/variety of godeffroyi)
- Wheeler, W. M. 1928d. Ants collected by Professor F. Silvestri in Japan and Korea. Boll. Lab. Zool. Gen. Agrar. R. Sc. Super. Agric. 22: 96-125 (page 115, subspecies/variety of godeffroyi)
- Yoshimura, M. and Onoyama, K. 2007. A new sibling species of the genus Strumigenys, with a redefinition of S. lewisi Cameron., Advances in ant systematics (Hymenoptera: Formicidae): Homage to E. O. Wilson - 50 years of contributions. (Memoirs of the American Entomological Institute 80). pp. 664-690.
References based on Global Ant Biodiversity Informatics
- Abe T., and A. Maeda. 1977. Fauna and density of ants in sugarcane fields of the southern part of Okinawa Island. Pp. 75-91 in: Ikehara, S. (ed.) 1977. Ecological studies of nature conservation of the Ryukyu Islands - (III). Naha, Okinawa: University of the Ryukyus, 202 pp.
- Arakelian G. R., and G. M. Dlussky. 1991. Dacetine ants (Hymenoptera: Formicidae) of the USSR. [In Russian.]. Zoologicheskii Zhurnal 70(2): 149-152.
- Azuma M. 1977. On the myrmecological-fauna of Mt. Rokko, Hyogo, with description of a new species (Formicidae, Hymenoptera). Hyogo Biology 7:112-118.
- Azuma, S. and M. Kinjo. 1987. Family Formicidae, In Checklist of the insects of Okinawa. The Biological Society of Okinawa, Nishihara. Pages 310-312.
- Bharti H. 2011. List of Indian ants (Hymenoptera: Formicidae). Halteres 3: 79-87.
- Bolton, B. 2000. The Ant Tribe Dacetini. Memoirs of the American Entomological Institute 65
- Brown W. L., Jr. 1949. Revision of the ant tribe Dacetini. I. Fauna of Japan, China and Taiwan. Mushi 20: 1-25.
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Choi B.-M. 1987. Taxonomic study on ants (Formicidae) in Korea (1). On the genus Monomorium. Journal of the Institute of Science Education (Cheongju National Teachers' College) 11:17-30.
- Choi B.M. 1986. Studies on the distribution of ants (Formicidae) in Korea. Journal of Chongju National Teacher College 23: 317-386.
- Choi B.M. 1988. Studies on the distribution of ants (Formicidae) in Korea (5) Ant fauna in Is. Kanghwado. Chongju Sabom Taehak Nonmunjip (Journal of Chongju National Teacher' College) 25: 217-231.
- Choi B.M. 1995. Taxonomic study on ants (Tribe: Dacetini) in Korea. Korean J. Entomol. 25(3): 189-196.
- Choi B.M. 1996. Distribution of ants (Formicidae) in Korea (16) - Ant fauna from Chollabukdo. Korean J. Soil. Zoology 1(1): 5-23.
- Choi B.M. 1996. Distribution of ants (Formicidae) in Korea (16): Ant fauna from Chollabukdo. Korean Journal of Soil Zoology 1(1): 5-23.
- Choi B.M. 1996. Studies on the distribution of ants (Formicidae) in Korea (15) -Ant fauna islands Ullungdo and Dokdo. Journal of Chongju National University of Education 33: 201-219.
- Choi B.M. 1997. Distribution of Ants (Formicidae) in Korea (18). Ants Fauna in island Paekryongdo and Taechongdo. Journal of Chongju National University of Education 34: 119-138.
- Choi B.M. 1998. Distribution of ants (Formicidae) in Korea (19) Ants fauna from Chungcheongbukdo Province. Cheongju Tea. Coll. 35: 213-266.
- Choi B.M., Bang, J.R. 1992. Studies on the distribution of ants (Formicidae) in Korea (9). Ant fauna in Mt. Togyusan. Korean Journal of Applied Entomology 31:101-112.
- Choi B.M., I. H. Lee. 1995. Studies on the distribution of ants (Formicidae) in Korea (14). Ant fauna in island Sohuksando. Korean Journal of Applied Entomology 34(3): 191-197.
- Choi B.M., K. Ogata, and M. Terayama. 1993. Comparative studies of ant faunas of Korea and Japan. 1. Faunal comparison among islands of Southern Korean and northern Kyushu, Japan. Bull. Biogeogr. Soc. Japan 48(1): 37-49.
- Choi B.M., Kim, C.H., Bang, J.R. 1993. Studies on the distribution of ants (Formicidae) in Korea (13). A checklist of ants from each province (Do), with taxonomic notes. Cheongju Sabom Taehakkyo Nonmunjip (Journal of Cheongju National University of Education) 30: 331-380.
- Choi B.M., and H.S. Lee. 1999. Studies on the distribution ants in Korea (21) - Ant fauna in Kwanaksan. Korean J. Soil Zoology 4(1): 1-4.
- Choi B.M., and J. R. Bang. Studies on the distribution of ants (Formicidae) in Korea (12): the analysis of ant communities in 23 islands. Journal of Cheongju National University of Education 30:317-330.
- Choi B.M., and Park, K.S. 1991. Studies on the distribution of ants (Formicidae) in Korea (7). Ant fauna in Mt. Kyeryongsan. Korean Journal of Applied Entomology 30: 80-85.
- Eguchi K.; Bui T. V.; Yamane S. 2011. Generic synopsis of the Formicidae of Vietnam (Insecta: Hymenoptera), part I Myrmicinae and Pseudomyrmecinae. Zootaxa 2878: 1-61.
- Emery C. 1897. Formicidarum species novae vel minus cognitae in collectione Musaei Nationalis Hungarici quas in Nova-Guinea, colonia germanica, collegit L. Biró. Természetrajzi Füzetek 20: 571-599.
- Eto S., and K. Ogata. 1983. Ants of Hirado Island, Kyushu. Bulletin of the Nagasaki Prefecture Biological Group 25: 7-11.
- Forel A. 1903. Les Formicides de l'Empire des Indes et de Ceylan. Part X. J. Bombay Nat. Hist. Soc. 14: 679-715.
- Fukumoto S. and Sk. Yamane. 2015. Records of ants from Uke–jima, Amami Islands, Japan (Hymenoptera, Formicidae). Nature of Kagoshima 41: 195–197.
- Fukumoto S., Jaitrong W. and Yamane S.K. 2013. Ant Fauna of Kuro-shima, Iwo-jima and Take-shima islands, Kagoshima Prefecture, southwestern Japan. Nature of Kagoshima 39: 119-125
- Fukumoto S., R. Satria, T. Maeda, and S. Yamane. 2014. Ant fauna of Gaja-jima, Tokara Islands, southwestern Japan. Nature of Kagoshima 40: 127131.
- Fukumoto S., Sk. Yamane, and M. Hira. 2016. Records of ants from Yoro-Shima, Amami Gunto, Japan (Hymenoptera, Formicidae). Nature of Kagoshima 42: 461–464.
- Fukumoto S., W. Jaitrong, and S. Yamane. 2013. Ant fauna of Take-shima, Iwo-jima and Kuro-shima islands, Kagoshima Prefecture, southwestern Japan. Nature of Kagoshima 39: 99-105.
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Ha S.J, S.J. Park, and B.J. Kim. 2002. Comparative ant faunas between Seonyudo and seven other islands of West Sea in Korea. Korean Journal of Entomology 32(2): 75-79.
- Harada Y. 1997. Ants from the Koshiki islands, Kagoshima-ken, southern Japan. Ari 21: 1-4.
- Harada Y. 2000. Ant fauna of the forest floor of the Koshikijima Islands, Kagoshima-ken, southern Japan. Ari 24: 4-11.
- Harada Y. S. Koto, N. Kawaguchi, K. Sato, T. Setoguchi, R. Muranaga, H. Yamashita, A. Yo, and S. Yamane. 2012. Ants of Jusso, Isa City, Kagoshima Prefecture, southwestern Japan. Bull. biogeogr. Soc. Japan 67: 143-152.
- Harada Y., D. Fukukura, R. Kurisu, and S. Yamane. 2013. Ants of Ports, monitoring of alien ant species. Bull. Biogeogr. Soc. Japan 68: 29-40.
- Harada Y., H. Yadori, M. Yoneda, R. Takinami, K. Nagahama, Y. Matsumoto, A. Oyama, S. Maeda, and S. Yamane. 2009. Ant fauna of Tanegashima (Hymenoptera, Formicidae). Nankiseibutu, the Nanki Biological Society 51(1): 15-21.
- Harada Y., K. Nishikubo, K. Matsumoto, M. Matsuda, Y. Inazawa, Y. Ozono, S. Koto, N. Kawaguchi, and S. Yamane. 2011. Ant fauna of Japanese beech (Fagus crenata) forests in southwestern Japan. Bull. Biogeogr. Soc. Japan 66: 115-127.
- Harada Y., K. Tashiro, K. Ebihara, H. Yadori, M. Yoneda, R. Takinami, K. Nagahama, and K. Hayashi. 2008. Ant fauna of the lavas of Sakurajima Volcano, Southern Japan. Bull. Biogeogr. Soc. Japan 63: 205-215.
- Harada Y., M. Enomoto, K. Nishimuta, and H. Mizumata. 2015. Ants of the Amami Islands, central Ryukyus, Japan. Nature of Kagoshima 41: 199–208.
- Harada Y., M. Enomoto, N. Nishimata, and K. Nishimuta. 2014. Ants of the Tokara Islands, northern Ryukyus, Japan. Nature of Kagoshima 40: 111121.
- Harada Y., Y. Matsumoto, S. Maeda, A. Oyama, and S. Yamane. 2009. Comparison of ant fauna among different habitats of Yaku-shima Island, southern Japan. Bull. Biogeogr. Soc. Japan 64: 125-134.
- Harada Y., Yadori H., Takinami R., Nagahama K., Matsumoto Y., Oyama A., Maeda S. and Yamane S.K. 2013. Ants of the southernmost Fagus crenata forest in Japan. Nature of Kagoshima 39: 113-118
- Hisamatsu M. 2004. List of Hymenoptera Recorded in Ibaraki Prefecture. Bulletin of Ibaraki Nature Museum 7: 125-164.
- Hosoichi S., M. M. Rahman, T. Murakami, S. H. Park, Y. Kuboki, and K. Ogata. 2019. Winter activity of ants in an urban area of western Japan. Sociobiology 66(3): 414-419.
- Hosoichi S., M. Yoshimura, Y. Kuboki, and K. Ogata. 2007. Ants from Yakushima Island, Kagoshima Prefecture. Ari 30: 47-54.
- Hosoichi S., W. Tasen, S. H. Park. A. Le Ngoc, Y. Kuboki, and K. Ogata. 2015. Annual fire resilience of ground-dwelling ant communities in Hiraodai Karst Plateau grassland in Japan. Entomological Science 18: 254–261.
- Hosoishi S. 2006. Ant fauna of Noko Island. pp99-107. In: The floristic and faunistic surveys of the Noko Island.
- Hosoishi S., M. Yoshimura, Y. Kuboki, and K. Ogata. 2007. Ants from Yakushima Island , Kagoshima Prefecture. Ari 30: 47-54.
- Ikeshita Y., A. Gotoh, K. Yamamoto, N. Taniguchi, and F. Ito. 2007. Ants collected in Mt. Linoyama, Marugame, Kagawa Prefecture (Hymenoptera, Formicidae). Kagawa Seibutsu 34: 59-62.
- Katayama M., T. Hosoya, and W. Toki. 2013: First survey of ground-dwelling ants (Hymenoptera: Formicidae) on the uninhabitedGaja-jima Island, theRyukyu archipelago, Japan.Entomol. Fennica 24: 216222.
- Kawahara Y., S. Hosoyamada, and S. Yamane. 1999. Ant fauna of the Terayama Station for Education and Research on Nature, Kagoshima University. Bulletin of the Faculty of Education, Kagoshima University. Natural Science 50: 147-156.
- Kim B., Ryu D., Park S., and J. Kim. 1994. Systematic study on ants from coasts of Korean Peninsula (Hym: Formicidae). Korean journal of entomology 24: 293-309.
- Kim B.J. 1996. Synonymic list and distribution of Formicidae (Hymenoptera) in Korea. Entomological Research Bulletin Supplement 169-196.
- Kim B.J., K.G. Kim, D.P. Ryu, J.H. Kim. 1995. Ants of Chindo island in Korea (Hymenoptera; Formicidae). The Korean Journal of Systematic Zoology 11(1): 101-113.
- Kim B.J., K.G. Kim, J.H. Kim, S.J. Park. Ants from Mt. Mirok. Korean J. Soil Zoology 2(2): 115-128.
- Kim B.J., S.J. Park, and J.H. Kim. 1996. Ants from Naejangsan national park (Hymenoptera: Formicidae). Korean J. Soil. Zoology &(2): 120-133.
- Kim B.J.; Kim, K.G.; Lim, K.H. 1993. Systematic study of ants from Chejudo province. Korean Journal of Entomology 23: 117-80
- Kim B.J.; Kim, K.G.; Lim, K.H. 1993. Systematic study of ants from Chejudo province. Korean Journal of Entomology 23: 117-81
- Kim C.H., B.M. Choi, and J.R. Bang. 1992. Studies on the distribution of ants (Formicidae) in Korea (8)-Ant fauna in 10 islands, Chollanam-do. Korean J. Appl. Entomol. 31(4): 345-359.
- Kim K.I., C.H. Kim, and B. Choi. 1989. The ant fauna of the southern shore in Gyeongsangnamdo, Korea. Journal of Gyeongsang Nat. Univ. 28(2): 213-226.
- Kim et al. 1993. Systematic study of ants from Chejudo Province. Koran Journal of Entomology 23(3): 117-141.
- Kim, Byung-Jin, Ky-Gyong Kim, Dong-Pyo Ryu and Joong-Hyon Kim. 1995. The Korean Journal of Systematic Zoology. 11(1):101-113.
- Kondoh M., and Y. Kitazawa. 1984. Ant communities of the campus of UOEH and in an adjacent natural forest. Journal of UOEH 6(3): 221-234.
- Kubota. S., and M. Terayama. 1988. Ant fauna of Tokyo. (1) A list of ants collected at the parks. ARI Reports of the Myrmecologists Society (Japan) 16: 14-16
- Kwon T. S. 2012. Korean ant atlas. Korea Forest Research Institute 162 pages.
- Kwon T. S. 2015. Ant assemblages along the Baekdudaegan Mountain Range in South Korea: Human roads and temperature niche. Journal of Asia-Pacific Biodiversity 8: 152-157.
- Kwon T. S. 2018. High competition between ant species at intermediate temperatures. Journal of Thermal Biology 72: 59-66.
- Kwon T. S., S. S. Kim, and J. H. Chun. 2014. Pattern of ant diversity in Korea: An empirical test of Rapoport's altitudinal rule. Journal of Asia-Pacific Entomology 17: 161167.
- Kwon T.S., C. M. Lee, J. H. Chun, J. H. Sung, and S. K. Kim. 2011. Ants in Hongneung forest. Korea Forest Research Institute, 92 pages.
- Li X., D. Hao, and Y. Huang. 2011. Ant species diversity at piedmont of Zijin Mountain in Nanjing. Journal of Nanjing Forestry University ( Natural Science Edition) 35(5): 55-58.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Lyu D.P. 2003. Systematics of Myrmicinae from Korea (Hymenoptera: Formicidae). PhD thesis Faculty of the Graduate School of Chungbuk National University 330 pages.
- Lyu D.P., B.M. Choi, and S. Cho. 2001. Review of Korean Dacetini (Hymenoptera: Formicidae: Myrmicinae). Ins. Koreana 18(3): 1-13.
- Maeto K. and S. Sato. 2004. Impacts of forestry on ant species richness and composition in warm-temperate forests of Japan. Forest Ecology and Management 187: 213223.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (First report; Ants of lowland). Ari 17: 6.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (first report; ants of lowland). Ari 18: 6.
- Manabe K. 1994. Ants of the shrine forest in Fukuoka Prefecture (second report; ants of mountain zone). Ari 17: 8.
- Masuko K. 1982. A data on the myrmecofauna of Miyake Island. Ari 10: 1-2.
- Masuko K. 1984. Studies on the predatory biology of Oriental Dacetine ants (Hymenoptera: Formicidae) I. Some Japanese species of Strumigenys, Pentastruma, and Epitritus, and a Malaysian Labidogenys, with special reference to hunting tactics in short-mandibulate forms. Insectes Sociaux 31(4): 429-451.
- Matsumura S. and Yamane Sk. 2012. Species composition and dominant species of ants in Jigenji Park, Kagoshima City, Japan. Nature of Kagoshima 38: 99107
- Matsumura S., and S. Yamane. 2012. Species composition and dominant species of ants in Jigenji Park, Kagoshima City, Japan. Nature of Kagoshima 38: 99-107.
- Menozzi C. 1940. Contribution à la faune myrmécologique du Japon. Mushi. 13: 11-12.
- Minato M., T. Kameyama, F. Ito, and T. Itino. 1996. A preliminary report of ant fauna in Gagawa Prefecture. Ari 20: 9-13.
- Miyama D., S. Yamane, T. Hishida, T. Kyono, T. Saito, T. Kuwahara, and N. Inoue. 2007. Ant Fauna in a Coppice Area around Shishitsuka-Ohike in Tsuchiura, Central Japan (Hymenoptera, Formicidae). Bulletin of Ibaraki Nature Museum 10: 1-10.
- Mizota K. 2002. A check list of insects in Kinkazan Island, Miyagi Pref., Northeastern Japan: A bibliographical Survey. Bulletin of Miyagi University of Education Environmental Education 5: 69-78.
- Ogata K. 1981. The ant fauna of the Goto islands, Natural history of the Goto? Islands, Japan : Iki Tsushima to no taihi (Danjo Gunto? Ko?rai Sone o fukumu Japan: 347-351.
- Our results Winkler 2009
- Paik W.H. 1984. A checklist of Formicidae (Hymenoptera) of Korea. Korean J. Plant Prot. 23(3): 193-195.
- Park S. H., S. Hosoishi, K. Ogata, and Y. Kuboki. 2014. Clustering of ant communities and indicator species analysis using self-organizing maps. Comptes Rendus Biologies http://dx.doi.org/10.1016/j.crvi.2014.07.003
- Park S.J., and B.J. Kim. 2002. Faunal comparison of ants among Cheongsando and other islands of South Sea in Korea. Korean Journal of Entomology 32(1): 7-12.
- Park, Seong, Joon and Byung, and Kim, Jin. 2002. Faunal Comparison of Ants among Cheongsando and Other Islands of South Sea in Korea. Korean Jornal of Entomology. 32(1):7-12.
- Radchenko, A. 2005. Monographic revision of the ants (Hymenoptera, Formicidae) of North Korea. Annales Zoologici 55(2): 127-221.
- Sato T., N. Tsurusaki, K. Hamaguchi, and K. Kinomura. 2010. Ant fauna of Tottori prefecture, Honshu, Japan. Bulletin of the Tottori Prefectural Museum 47: 27-44.
- Shimazaki, A. and Miyashita, T. 2005. Variable dependence on detrital and grazing food webs by generalist predators: aerial insects and web spiders. Ecography 28: 485-495.
- Shimono A., and S. Yamane. 2003. Ant species diversity on Okinoerabu-jima, the Ryukyus, southern Japan. For the Establishment of Remote Islands Study (Kagoshima Univ.) 3: 11-29.
- Takechi F. 1960. A list of ants unrecorded from Mt. Ishizuchi and Omogo Valley, Iyo, Shikoku (Hymenoptera: Formicidae). Transactions of the Shikoku Entomological Society 6:91.
- Tanaka H. O., T. F. Haraguchi, I. Tayasu, and F. Hyodo. 2019. Stable and radio-isotopic signatures reveal how the feeding habits of ants respond to natural secondary succession in a cool-temperate forest. Insectes Sociaux 66(1): 37-46.
- Terayama M. 1977. Checklist of the known ants of Saitama Prefecture. Insects and nature 12(4): 26-27
- Terayama M. 1983. Kagoshima-ken-hondo no ari. Kanagawa-chucho (Journal of the Kanagawa Entomologists Association): 13-24.
- Terayama M. 1992. Structure of ant communities in East Asia. A. Regional differences and species richness. Bulletin of the Bio-geographical Society of Japan 47: 1-31.
- Terayama M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta: Hymenoptera). Research Bulletin of Kanto Gakuen University. Liberal Arts 17:81-266.
- Terayama M., Choi, B.M., Kim, C.H. 1992. A check list of ants from Korea, with taxonomic notes. Bulletin of the Toho Gakuen 7:19-54.
- Terayama M., K. Ogata, and B.M. Choi. 1994. Distribution records of ants in 47 prefectures of Japan. Ari (report of the Myrmecologists Society of Japan) 18: 5-17.
- Terayama M., S. Kubota, and K. Eguchi. 2014. Encyclopedia of Japanese ants. Asakura Shoten: Tokyo, 278 pp.
- Terayama M., and K. Murata. 1987. Relation between ant communities and vegetations of Toshima island, the Izu Islands. Bull. Biogeogr. Soc. Japan 42(9): 57-63.
- Terayama M., and K. Murata. 1990. Effects of area and fragmentation of forests for nature conservation: Analysis by ant communities. Bull. Biogeogr. Soc. Japan 45(2): 11-17.
- Terayama M., and S. Kubota. 1994. Ants from Aogashima Island. Ari 17: 11.
- Terayama M., and S. Kubota. 2002. Ants of Tokyo, Japan. ARI 26: 1-32.
- Terayama M., and S. Yamane. 1984. Ants of Yaku-shima Island, the northern Ryukyus, with reference to their altitudinal distribution (Insecta: Hymenoptera). Cons. Rep. Yaku-shima Wildness Area, Kyushu, Japan, pp. 643-667. Nat. Cons. Bureau, Env. Agency, Japan.
- Terayama, M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta; Hymenoptera). The Research Bulletin of Kanto Gakuen University 17: 81-266.
- Terayama. M. 2004. Geological and ecological distribution of Japanese ants communities. (translated from Japanese) Reports of the Saitama Prefecture Animal Research Association. 48:32
- Teruyama. M. 1988. Ant fauna of Saitama Prefecture, Japan. ARI Reports of the Myrmecologists Society (Japan) 16: 4-13
- Tsurusaki N., Y. Kawakami, K. Ichisawa, M. Hayashi, and R. Miyanaga. 2012. Insect fauna of Kamogaiso Beach of the Uradome Coast, Tottori Prefecture, Japan. ??????? 7: 1-8.
- Wheeler W. M. 1906. The ants of Japan. Bulletin of the American Museum of Natural History 22: 301-328.
- Wheeler W. M. 1928. Ants collected by Professor F. Silvestri in China. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 22: 3-38.
- Wheeler W. M. 1928. Ants collected by Professor F. Silvestri in Japan and Korea. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 22: 96-125.
- Wheeler W. M. 1930. A list of the known Chinese ants. Peking Natural History Bulletin 5: 53-81.
- Xu Z. 1999. [An analysis on the ant fauna of the tropical rain forest in Xishuangbanna of China.] Zoological Research 20: 379-384.
- Yamane S. 2016. How many species of Ants in Amami Islands? (in Japanese). Part 2, chapter 1 in How many species of Ants in Amami Islands? Pp. 92-132.
- Yamane S. S. Fukumoto, Y. Maeda, and Y. Sato. 2017. Records of ants from Kakeroma-jima, the Amami Islands, Japan. Bull. Biogeogr. Soc. Japan 71, 131-137.
- Yamane S., S. Ikudome, and M. Terayama. 1999. Identification guide to the Aculeata of the Nansei Islands, Japan. Sapporo: Hokkaido University Press, xii + 831 pp. pp, 138-317.
- Yamane S., Y. Harada, and K. Eguchi. 2013. Classification and ecology of ants. Natural history of ants in Southern Kyushu. 200 pages
- Yamane S.; Ikudome, S.; Terayama, M. 1999. Identification guide to the Aculeata of the Nansei Islands, Japan. Sapporo: Hokkaido University Press, xii + 831 pp. pp138-317.
- Yoshimura M., and K. Onoyama. 2007. A new sibling species of the genus Strumigenys, with a redefinition of S. lewisi Cameron. Memoirs of the American Entomological Institute 80:664-690.
- Yoshimura M., and K. Onoyama. 2008. Taxonomic changes in the genus Strumigenys of Japan from 2003. Ari 31: 1-12.
- Yoshitomi H., and S. Matsuno. 2012. List of species of Hymenoptera and Diptera in Matsuyama City, Ehime Prefecture, Shikoku, Japan. pp. 167-176. In: Committee for Surveys of Natural Environment of Matsuyama City (Chief Editor: Kazuo ISHIKAWA) (ed.) Checklist of the Wild Animals, Fungi, and Plants of Matsuyama City, 2012. Published by the Department of Environment, Matsuyama City, 404 pp.
- Zhang N. N., Y. Q. Chen, Z. X. Lu, W. Zhang, and K. L. Li. 2013. Species diversity, community structure difference and indicator species of leaf-litter ants in rubber plantations and secondary natural forests in Yunnan, southwestern China. Acta Entomologica Sinica 56(11): 1314-1323.
- Zhou S.-Y. and Xu Z. 2003. Taxonomic study on Chinese members of the ant genus Strumigenys F. Smith (Hymenoptera, Formicidae) from the mainland of China. Acta Zootaxonomica Sinica28(4): 737-740.

