Paltothyreus tarsatus
| Paltothyreus tarsatus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Paltothyreus |
| Species: | P. tarsatus |
| Binomial name | |
| Paltothyreus tarsatus (Fabricius, 1798) | |
| Subspecies | |
| |
| Synonyms | |
| |
The African Stink Ant is a large (17-20 mm) conspicuous predator and scavenger distributed throughout Africa. The populous colonies inhabit ground nests with multiple entrances, revealing an extensive system of shallow tunnels that lead to the foraging grounds (Braun et al. 1994), the entrance often surrounded by excavated soil and remains of arthropods and other food. The characteristic "rotten egg" smell is caused by mandibular gland secretions. These sulphides are released by workers that become buried after tunnels collapse, and this allows nestmates to locate and excavate them (Crewe & Fletcher 1974). Workers usually forage singly. In Benin, Taylor et al. (2018) found it on mango (Mangifera indica) and as prey in nests of Oecophylla longinoda.
Photo Gallery
 Paltothyreus tarsatus biting onto a green stem. Once infected by an Ophiocordyceps species (still undescribed), it climbs up to a vertical stem to die biting onto it. In this case, the fungus we see is actually a hyerparasite of the Ophiocordyceps fungus, which overcame it and procuced these torrubielloid sexual structures. (Photo by João P. M. Araújo)
Paltothyreus tarsatus biting onto a green stem. Once infected by an Ophiocordyceps species (still undescribed), it climbs up to a vertical stem to die biting onto it. In this case, the fungus we see is actually a hyerparasite of the Ophiocordyceps fungus, which overcame it and procuced these torrubielloid sexual structures. (Photo by João P. M. Araújo)
Identification
There are currently a number of subspecies recognized (P. tarsatus delagoensis [Emery], P. tarsatus mediana [Santschi], P. tarsatus robusta [Santschi], P. tarsatus striata [Santschi], P. tarsatus striatidens [Santschi] and P. tarsatus subopaca [Santschi]) and possibly some of these may be recognized as valid species when the Old World fauna is evaluated.
From Mackay and Mackay (2010): Paltothyreus tarsatus can be easily separated from all of the New World species by the angulate anterior lateral corners of the postpetiole and the presence of single small teeth on each of the inner margins of the tarsal claws. The specimen from São Paulo is nearly identical to the typical Old World Paltothyreus tarsatus . It can be differentiated by the form of the surface of the medial lobe of the clypeus (which is completely concave with notable longitudinal striae in most Old World P. tarsatus), smooth surface of the mandible (striate and dull in Old World P. tarsatus), the transverse striae on the mesonotum (longitudinal in most Old World P. tarsatus) and by the smooth and glossy dorsal surface of the gaster (smooth and glossy, but with scattered coarse punctures in most Old World specimens of P. tarsatus). It is probable that this specimen of P. tarsatus is a mislabeled Old World species, but it may also represent a new species.
It is interesting to note that the striae on the dorsum of the pronotum are very similar to those of Pachycondyla magnifica (=Neoponera magnifica). The two species are apparently not closely related and easily separated by the claw on the inner border of the tarsal claw and the angles on the postpetiole of P tarsatus, both of which are absent in P. magnifica.
Distribution
From Mackay and Mackay (2010): Smith (1858:94) lists P. tarsatus as being a South American species (Demerara, Pará, Brasil). Unfortunately Neoponera commutata was misidentified as P. tarsatus prior to 1860 (Roger, 1860), which explains Smith’s report.
Latitudinal Distribution Pattern
Latitudinal Range: 13.447675° to -32.360532°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Afrotropical Region: Benin, Cameroun, Democratic Republic of Congo, Equatorial Guinea, Gambia, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Mozambique, Namibia, Nigeria, Senegal (type locality), Sierra Leone, South Africa, Uganda, United Republic of Tanzania, Zimbabwe.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
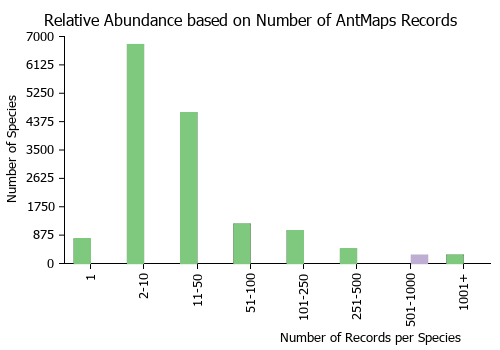
|
Biology
Although workers of P. tarsatus forage individually, they can recruit nestmates with chemical signals (sternal glands in abdomen) when retrieving bigger or more abundant prey items (Hölldobler 1984). Workers can transfer prey to nestmates during the journey back to the nest (López et al., 2000). Records of prey carried by foragers as well as prey remains outside nest entrances show more than 90 % arthropods, especially termites (Macrotermes) and millipedes, but also ants (mainly Camponotus sp.) and beetles. Examination of 40 complete colonies from savanna and forest habitats in Ivory Coast indicated a mean colony size of 1576 ± 1543 workers (n = 42), and three colonies yielded more than 5000 workers (Peeters et al. 2013). There was a huge quantity of brood, exceeding 1000 eggs and several thousands of larvae in some colonies. Five colonies had more than 1000 cocoons. Some of the bigger colonies (>1000 workers) reared hundreds of winged gynes, with a maximum of 823. Only a few other species scattered throughout subfamily Ponerinae have thousands of workers per colony, e.g. Brachyponera lutea, Centromyrmex bequaerti, Megaponera analis, Neoponera luteola.
Nests are decentralized to allow harvesting food throughout a large home range while reducing predation on foragers. The subterranean tunnels lead foragers away from the nest centre; once on the surface, they do not roam more than 3–5 m from an exit. They use canopy orientation during excursions away from the tunnel exits (Hölldobler 1980).
Paltothyreus tarsatus has volatile material in the venom gland, which includes bitter-tasting cyclic dipeptides (Morgan et al., 2003).
Diame et al. (2015) - An ant diversity study in Senegal orchards found Paltothyreus tarsatus occurred in and preferred high canopy coverage, higher tree diversity and more leaf litter.
Mating and colony foundation
In the Ivory Coast, the mating behaviour of P. tarsatus follows the ‘male-aggregation’ syndrome which is uncommon in Ponerinae (Life History). In contrast, Villet et al. (1989) described a ‘female calling’ syndrome for P. tarsatus in South Africa, where gynes emerged from a nest entrance, climbed low vegetation nearby, and adopted a resting posture; after some time males flew in and mated. Female-calling resembles the sexual behaviour of solitary wasps and may be ancestral in ants (Hölldobler & Bartz 1985). Male-aggregations seem possible only in ponerine species with big colonies and numerous sexuals produced concurrently. The intraspecific variation documented in P. tarsatus suggests that a transition from female-calling to male-aggregation is possible upon an increase in the number of sexuals in the population (Peeters et al. 2013). After mating, ant foundresses are alone for several weeks while they raise the first generation of workers. Foundations are especially hazardous in the Ponerinae because queens must hunt outside the nests and produce workers that are similar in size to themselves (no nanitics) (Life History). This is unlike formicoid subfamilies in which claustral foundresses can rely on histolysis of their big flight muscles and other metabolic reserves. In P. tarsatus, non-claustral foundation has been observed in South Africa (Villet et al. 1989) and in the Ivory Coast. Founding queens presumably have low success rates due to predation while foraging as well as parasitism of unguarded brood. Hence, many gynes must be produced annually to compensate for mortality during both dispersal and foundation itself.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a associate (details unknown) for the fungus Gibellula carnata (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Gibellula liberiana (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Gibellula liberiana (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Pseudogibellula formicarum (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Sporothrix insectorum (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the fungus Verticillium nodulosum (a associate (details unknown)) (Quevillon, 2018).
- This species is a host for the fungus Akanthomyces gracilis (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Akanthomyces gracilis (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Ophiocordyceps sp. (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Polycephalomyces cylindrosporus (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Stilbella buquetti var. formicarum (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Ophiocordyceps australis (a pathogen) (Shrestha et al., 2017).
- This species is a host for the fungus Cordyceps carnata (a pathogen) (Shrestha et al., 2017).
- This species is a host for the fungus Ophiocordyceps myrmecophila (a pathogen) (Shrestha et al., 2017).
Castes
Winged queens and workers exhibit a pronounced dimorphism in body size.
Worker
  
| |
| . | Owned by Museum of Comparative Zoology. |
Images from AntWeb
   
| |
| Worker. Specimen code casent0172430. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ANIC, Canberra, Australia. |
   
| |
| Worker (major/soldier). Specimen code sam-hym-c000249a. Photographer Hamish Robertson, uploaded by California Academy of Sciences. | Owned by SAMC, Cape Town, South Africa. |
Queen
   
| |
| . | |
Male
   
| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- tarsatus. Formica tarsata Fabricius, 1798: 280 (w.) SENEGAL. Latreille, 1802c: 736 (q.); Mayr, 1866b: 893 (m.). Combination in Paltothyreus: Mayr, 1862: 736; in Pachycondyla: Brown, in Bolton, 1995b: 310; in Paltothyreus: Schmidt & Shattuck, 2014: 127. Senior synonym of gagates, pestilentia, spiniventris: Roger, 1860: 310; Roger, 1863b: 17; of simillima: Emery, 1892d: 557. Current subspecies: nominal plus delagoensis, medianus, robustus, striatidens, striatus, subopacus. See also: Forel, 1891b: 136; Arnold, 1915: 44; Wheeler, W.M. 1922a: 60; Hölldobler, 1980: 86; Mackay & Mackay, 2010: 545.
- gagates. Ponera gagates Guérin-Méneville, 1844a: 423 (w.) SENEGAL. Junior synonym of tarsatus: Roger, 1860: 310; Roger, 1863b: 17.
- pestilentia. Ponera pestilentia Smith, F. 1858b: 92 (w.) SIERRA LEONE. Junior synonym of tarsatus: Roger, 1860: 310.
- simillima. Pachycondyla simillima Smith, F. 1858b: 105, pl. 7, fig. 17 (q.) SOUTH AFRICA. Combination in Paltothyreus: Mayr, 1886c: 358. Junior synonym of tarsatus: Emery, 1892d: 557.
- spiniventris. Ponera spiniventris Smith, F. 1858b: 92 (m.) SIERRA LEONE. Junior synonym of tarsatus: Roger, 1860: 310; Roger, 1863b: 17.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
From Mackay and Mackay (2010): The worker is a moderately large (total length 13 mm) dark reddish brown ant. The mandibles have approximately 18 teeth. The median portion of the clypeus is formed into a broad lobe, which overhangs the remainder of the clypeus. The surface of the lobe has two longitudinal depressions separating three distinct lobes (there are exceptions to this). The head is nearly square, with the length (including the lobe of the clypeus) being 3.1 mm, the width 3.0 mm. The eyes are relatively small (maximum diameter 0.56 mm) located slightly more than one maximum diameter from the anterior margin of the head. The scape is relatively short (2.66 mm), extending about 1½ funicular segments past the posterior lateral corner of the head. The sides of the head are slightly narrowed anteriorly, angulate posteriorly, with the medial posterior margin concave. The mesonotum and propodeum are barely separated on the dorsum of the mesosoma, but the metanotal suture is well developed on the side. The propodeal spiracle is elongated. The petiole is relatively narrow when viewed in profile with a distinctly concave anterior face and a broadly rounded convex posterior face, the faces of which form a poorly defined dorsal face. The subpetiolar process is well developed and consists of a thickened triangular lobe. The anterior upper corners of the postpetiole (first gastral tergum) are swollen and angulate. The stridulatory file is apparently absent (pretergite can not be well seen in the specimen from São Paulo, but the stridulatory file is absent in Old World specimens of P. tarsatus). The dorsum of the pygidium is slightly concave. The arolia are not developed. The tarsal claws have a distinct tooth along in inner medial margin on both sides.
Erect hairs are abundant on the clypeus, especially along the anterior border, as they are on the dorsum of the head, the antennal scapes, the sides of the head, the posterior margin, the ventral surface of the head, the dorsum of the mesosoma, all surfaces of the petiole and all surfaces of the gaster; the hairs on the legs are mostly erect, or at least suberect. Appressed pubescence is sparse and noticeable only on the head and the gaster.
The mandibles are smooth and glossy with scattered punctures, the lobe of the clypeus has poorly defined longitudinal striae; the dorsum of the head has well-developed longitudinal striae, which diverge posteriorly. The dorsum of the pronotum has very coarse longitudinal striae, which pass transversely across the pronotum anteriorly and form longitudinal striae on the side the pronotum, the dorsum of the mesonotum has coarse transverse striae as does the dorsum of the propodeum, much of the side of the mesopleuron is smooth and glossy, but the upper region has fine striae, the sides of the propodeum have obliquely directed striae, the petiole is mostly smooth and glossy, but poorly defined striae are present on the sides and transverse striae are present across the dorsum of the node, the gaster is moderately smooth and glossy with scattered punctures.
Queen
From Mackay and Mackay (2010): See the discussion of the tarsatus species complex.
Male
From Mackay and Mackay (2010): See the discussion of the tarsatus species complex.
Type Material
Senegal, Latreille; Sierra Leon; South Africa (Mackay and Mackay 2010)
The following notes on F. Smith type specimens have been provided by Barry Bolton (details):
Ponera pestilentia
Holotype worker in The Natural History Museum. Labelled “Sierra Leone.” Data of original description gives D.F. Morgan as collector.
Ponera spiniventris
Male in The Natural History Museum, labelled “Afric. spiniventris Smith.” This is probably the holotype, but the data should be, “Sierra Leone, D.F. Morgan.” Smith stated, “in all probablility this is the male of P. pestilentia.”
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Etymology
The name of this species is derived from the Greek word tarsos, referring to the sole of the foot, presumably referring to the unusual form of the tarsal claw. (Mackay and Mackay 2010)
References
- Arnold, G. 1915. A monograph of the Formicidae of South Africa. Part I. Ponerinae, Dorylinae. Ann. S. Afr. Mus. 14: 1-159 (page 44, see also)
- Azevedo, D.L.O., Medeiros, J.da C., Araújo, A. 2021. Flexibility in the integration of environmental information by Dinoponera quadriceps Kempf during foraging. Revista Brasileira de Entomologia 65, e20210084 (doi:10.1590/1806-9665-rbent-2021-0084).
- Borowiec, L., Salata, S. 2018. Notes on ants (Hymenoptera: Formicidae) from Gambia (Western Africa). Annals of the Upper Silesian Museum in Bytom, Entomology 26 (online 010), 1-13 (doi:10.5281/ZENODO.1243767).
- Braun, U., Peeters, C. & Hölldobler, B. 1994. The giant nests of the African Stink Ant, Paltothyreus tarsatus (Formicidae, Ponerinae). Biotropica 26: 308-311.
- Brown, W. L., Jr. 1995a. [Untitled. Taxonomic changes in Pachycondyla attributed to Brown.] Pp. 302-311 in: Bolton, B. A new general catalogue of the ants of the world. Cambridge, Mass.: Harvard University Press, 504 pp. (page 310, combination in Pachycondyla)
- Crewe R.M. & Fletcher D.J.C. 1974. Ponerine ant secretions: The mandibular gland secretion of Paltothyreus tarsatus. J. Entomological Society of southern Africa 37: 291-298.
- Diame, L., R. Blatrix, I. Grechi, J. Y. Rey, C. A. B. Sane, J. F. Vayssieres, H. de Bon, and K. Diarra. 2015. Relations between the design and management of Senegalese orchards and ant diversity and community composition. Agriculture Ecosystems & Environment. 212:94-105. doi:10.1016/j.agee.2015.07.004
- Egbon, I.N., Osabuohien, I.P. 2022. First checklist, species richness and diversity of leaf-litter dwelling ants (Hymenoptera: Formicidae) in ancient Benin moat, Nigeria. Animal Research International 19(3): 4634–4642.
- Emery, C. 1892f [1891]. Voyage de M. Ch. Alluaud dans le territoire d'Assinie (Afrique occidentale) en juillet et août 1886. Formicides. Ann. Soc. Entomol. Fr. 60: 553-574 (page 557, Senior synonym of simillima)
- Esteves, F.A., Fisher, B.L. 2021. Corrieopone nouragues gen. nov., sp. nov., a new Ponerinae from French Guiana (Hymenoptera, Formicidae). ZooKeys 1074, 83–173 (doi:10.3897/zookeys.1074.75551).
- Fabricius, J. C. 1798. Supplementum entomologiae systematicae. Hafniae [= Copenhagen]: Proft and Storch, 572 pp. (page 280, worker described )
- Forel, A. 1891c. Les Formicides. [part]. In: Grandidier, A. Histoire physique, naturelle, et politique de Madagascar. Volume XX. Histoire naturelle des Hyménoptères. Deuxième partie (28e fascicule). Paris: Hachette et Cie, v + 237 pp. (page 136, see also)
- Hölldobler, B. 1980. Canopy orientation: a new kind of orientation in ants. Science (Wash. D. C.) 210: 86-88 (page 86, see also)
- Ḧolldobler, B. 1984. Communication during foraging and nest-relocation in the African Stink ant, Paltothyreus tarsatus Fabr. (Hymenoptera, Formicidae, Ponerinae). Zeitschrift fur Tierpsychologie. 65:40–52.
- Hughes, D.P., Araújo, J.P.M., Loreto, R.G., Quevillon, L., de Bekker, C., Evans, H.C. 2016. From so simple a beginning. In: Advances in Genetics. Elsevier BV (doi:10.1016/bs.adgen.2016.01.004).
- Lachaud, J.-P., Déjean, A. 1994. Predatory behavior of a seed-eating ant: Brachyponera senaarensis. Entomologia Experimentalis et Applicata 72(2), 145–155 (doi:10.1111/j.1570-7458.1994.tb01812.x).
- Latreille, P. A. 1802a. Histoire naturelle des fourmis, et recueil de mémoires et d'observations sur les abeilles, les araignées, les faucheurs, et autres insectes. Paris: Impr. Crapelet (chez T. Barrois), xvi + 445 pp. (page 736?, queen described)
- López, F., C. Agbogba and I. Ndiaye. 2000. Prey chain transfer behaviour in the African stink ant Pachycondyla tarsata Fabr. Insectes Sociaux 47:337-342.
- Mackay, W.P., Mackay, E.E. 2010. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae). Edwin Mellon Press, Lewiston.
- Mayr, G. 1862. Myrmecologische Studien. Verh. K-K. Zool.-Bot. Ges. Wien 12: 649-776 (page 736, Combination in Paltothyreus)
- Mayr, G. 1866b. Diagnosen neuer und wenig gekannter Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 16: 885-908 (page 893, male described)
- Mbenoun Masse, P.S., Ebolo, G.L.M., Titti, G.E., Mony, R. (2021) Ant species richness, abundance and functional groups along an elevation gradient in Central Cameroon Biodiversity Journal, 2021, 12 1.: 179–194. Biodiversity Journal 12, 179–194 (doi:10.31396/biodiv.jour.2021.12.1.179.194).
- Morgan, E., H. Jungnickel, S. Keegans, R. do Nascimento, J. Billen, B. Gobin and F. Ito. 2003. Comparative survey of abdominal gland secretions of the ant subfamily Ponerinae. Journal of Chemical Ecology 29:95-114.
- Peeters, C., Braun, U. & Hölldobler, B. 2013. Large colonies and striking sexual investment in the African stink ant, Paltothyreus tarsatus (subfamily Ponerinae). African Entomology 21(1): 9-14.
- Pekár, S., Haddad, C. 2011. Trophic strategy of ant-eating Mexcala elegans (Araneae: Salticidae): looking for evidence of evolution of prey-specialization. Journal of Arachnology 39, 133–138 (doi:10.1636/hi10-56.1).
- Richter, A., Boudinot, B.E., Hita Garcia, F., Billen, J., Economo, E.P., Beutel, R.G. 2023. Wonderfully weird: the head anatomy of the armadillo ant, Tatuidris tatusia (Hymenoptera: Formicidae: Agroecomyrmecinae), with evolutionary implications. Myrmecological News 33: 35-75 (doi:10.25849/MYRMECOL.NEWS_033:035).
- Roger, J. 1860. Die Ponera-artigen Ameisen. Berl. Entomol. Z. 4: 278-312 (page 310, Senior synonym of gagates, pestilenta and spiniventris)
- Roger, J. 1863b. Verzeichniss der Formiciden-Gattungen und Arten. Berl. Entomol. Z. 7(B Beilage: 1-65 (page 17, Senior synonym of gagates, pestilenta and spiniventris)
- Santschi, F. 1919b. Nouvelles Fourmis du Congo Belge du Musée du Congo Belge, à Tervueren. Revue Zoologique Africaine 7:79-91.
- Schmidt, C.A. & Shattuck, S.O. 2014. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817, 1–242 (doi:10.11646/zootaxa.3817.1.1).
- Shrestha B, Tanaka E, Hyun MW, Han JG, Kim CS, Jo JW, Han SK, Oh J, Sung JM, Sung GH. 2017. Mycosphere Essay 19. Cordyceps species parasitizing hymenopteran and hemipteran insects. Mycosphere 8(9): 1424–1442 (DOI 10.5943/mycosphere/8/9/8).
- Smith, F. 1858. Catalogue of hymenopterous insects in the collection of the British Muséum. Part VI. Formicidae. London: British Muséum, 216 pp.
- Taylor, B., Agoinon, N., Sinzogan, A., Adandonon, A., Kouaguou, Y. N., Bello, S., Wargui, R., Anato, F., Ouagoussounon, I., Houngbo, H., Tchibozo, S., Todjihounde, R., Vayssieres, J.F. 2018. Records of ants (Hymenoptera: Formicidae) from the Republic of Benin, with particular reference to the mango farm ecosystem. Journal of Insect Biodiversity 8(1): 6-29 (doi:10.12976/jib/2018.08.1.2).
- Troya, A., Marcineiro, F., Lattke, J.E. & Longino, J. 2022. Igaponera curiosa, a new ponerine genus (Hymenoptera: Formicidae) from the Amazon. European Journal of Taxonomy 823: 82–101 (doi:10.5852/ejt.2022.823.1817).
- Wheeler, W. M. 1922b. Ants of the American Museum Congo expedition. A contribution to the myrmecology of Africa. II. The ants collected by the American Museum Congo Expedition. Bull. Am. Mus. Nat. Hist. 45: 39-269 (page 60, see also)
References based on Global Ant Biodiversity Informatics
- André E. 1895. Formicides de l'Ogooué (Congo français). Rev. Entomol. (Caen) 14: 1-5.
- Arnold G. 1915. A monograph of the Formicidae of South Africa. Part I. Ponerinae, Dorylinae. Annals of the South African Museum 14: 1-159.
- Belshaw R., and B. Bolton. 1994. A survey of the leaf litter ant fauna in Ghana, West Africa (Hymenoptera: Formicidae). Journal of Hymenoptera Research 3: 5-16.
- Belshaw R., and B. Bolton. 1994. A survey of the leaf litter ant fauna in Ghana, West Africa (Hymenoptera: Formicidae). Journal of Hymenoptera Research. 3: 5-16.
- Bernard F. 1953. La réserve naturelle intégrale du Mt Nimba. XI. Hyménoptères Formicidae. Mémoires de l'Institut Français d'Afrique Noire 19: 165-270.
- Borowiec L., and S. Salata. 2018. Notes on ants (Hymenoptera: Formicidae) from Gambia (Western Africa). Annals of the Upper Silesian Museum in Bytom Entomology 26: 1-13.
- Braet Y., and B. Taylor. 2008. Mission entomologique au Parc National de Pongara (Gabon). Bilan des Formicidae (Hymenoptera) recoltes. Bulletin S. R. B. E./K.B.V.E. 144: 157-169.
- Davis L. R., and L. E. Alonso. 2007. Ant species collected from the Atewa Range Forest Reserve during the 2006 RAP survey. Pp 171-172. McCullough, J., L.E. Alonso, P. Naskrecki, H.E. Wright and Y. Osei-Owusu (eds.). 2007. A Rapid Biological Assessment of the Atewa Range Forest Reserve, Eastern Ghana. RAP Bulletin of Biological Assessment 47. Conservation International, Arlington, VA.
- Diame L., B. Taylor, R. Blatrix, J. F. Vayssieres, J. Y. Rey, I. Grechi, and K. Diarra. 2017. A preliminary checklist of the ant (Hymenoptera, Formicidae) fauna of Senegal. Journal of Insect Biodiversity 5(15): 1-16.
- Emery C. 1892. Voyage de M. Ch. Alluaud dans le territoire d'Assinie (Afrique occidentale) en juillet et août 1886. Formicides. Annales de la Société Entomologique de France 60: 553-574.
- Emery C. 1911. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125.
- Ewuim S. C., M. A. Badejo, and O. O. Ajayi. 1997. Ants of forest and fallow plots in Nigeria. Biotropica 29(1): 93-99.
- Fisher B. L. 2004. Diversity patterns of ants (Hymenoptera: Formicidae) along an elevational gradient on Monts Doudou in southwestern Gabon. Memoirs of the California Academy of Sciences 28: 269-286.
- Forel A. 1897. Ameisen aus Nossi-Bé, Majunga, Juan de Nova (Madagaskar), den Aldabra-Inseln und Sansibar, gesammelt von Herrn Dr. A. Voeltzkow aus Berlin. Mit einem Anhang über die von Herrn Privatdocenten Dr. A. Brauer in Marburg auf den Seychellen und von Herrn Perrot auf Ste. Marie (Madagaskar) gesammelten Ameisen. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 21: 185-208.
- Forel A. 1909. Fourmis du Musée de Bruxelles. Fourmis de Benguela récoltées par M. Creighton Wellman, et fourmis du Congo récoltées par MM. Luja, Kohl et Laurent. Annales de la Société Entomologique de Belgique 53: 51-73.
- Forel A. 1910. Zoologische und anthropologische Ergebnisse einer Forschungsreise im westlichen und zentralen Südafrika ausgeführt in den Jahren 1903-1905 von Dr. Leonhard Schultze. Vierter Band. Systematik und Tiergeographie. D) Formicidae. Denkschriften der Medizinisch-Naturwissenschaftlichen Gesellschaft zu Jena 16: 1-30.
- Forel A. 1911. Fourmis nouvelles ou intéressantes. Bull. Soc. Vaudoise Sci. Nat. 47: 331-400.
- Forel A. 1913. Quelques fourmis du Musée du Congo Belge (1). Annales de la Société Entomologique de Belgique 57: 347-359.
- Garcia F.H., Wiesel E. and Fischer G. 2013.The Ants of Kenya (Hymenoptera: Formicidae)Faunal Overview, First Species Checklist, Bibliography, Accounts for All Genera, and Discussion on Taxonomy and Zoogeography. Journal of East African Natural History, 101(2): 127-222
- Glover P. E. 1967. Notes on some ants in northern Somalia. East African Wildlife Journal 5: 65-73.
- Hita Garcia, F., G. Fischer, M.K. Peters, R.R. Snelling and H.W. Wagele. 2009. A preliminary checklist of the ants (Hymenoptera: Formicidae) of Kakamega Forest (Kenya). Journal of East African Natural HIstory 98(2): 147-165.
- IZIKO South Africa Museum Collection
- Kolo Y. 2006. Evaluation rapide des fourmis de la région de Boké, Guinée. In Wright, H.E. and J. McCullough et M.S. Diallo. (eds). 2006. A Rapid Biological Assessment of the Boké Préfecture, Northwestern Guinea. RAP Bulletin of Biological Assessment 41. Conservation International, Washington, DC.
- Kone M., S. Konate, K. Yeo, P. K. Kouassi, and K. E. Linsenmair. 2012. Changes in ant communities along an age gradient of cocoa cultivation in the Oumé region, central Côte dIvoire. Entomological Science 15: 324339.
- Levieux J. 1972. Etude du peuplement en fourmis terricoles d'une savane preforestiere de Cote d'Ivoire. Revue d'Ecologie et de Biologie du Sol 10(3): 381-428.
- Levieux J., and T. Diomande. 1985. Evolution des peuplements de fourmis terricoles selon l'age de la végétation dans une foret de Cote d'Ivoire intacte ou soumise à l'action humaine. Insectes Sociaux 32(2): 128-139.
- Lévieux J. 1972. Les fourmis de la savane de Lamto (Côte d'Ivoire): éléments de taxonomie. Bulletin de l'Institut Fondamental d'Afrique Noire. Série A. Sciences Naturelles 34: 611-654.
- Lévieux J. 1977. La nutrition des fourmis tropicales: V- Elements de synthèse. Les modes d'exploitation de la biocenose. Insectes Sociaux 24(3): 235-260.
- Medler J. T. 1980: Insects of Nigeria - Check list and bibliography. Mem. Amer. Ent. Inst. 30: i-vii, 1-919.
- Menozzi C. 1930. Formiche della Somalia italiana meridionale. Memorie della Società Entomologica Italiana. 9: 76-130.
- Menozzi C. 1932. Raccolte mirmecologiche dell'Africa orientale conservate nel Museo Civico di Storia Naturale Giacomo Doria di Genova. Parte II. Formiche dell'Uganda e delle isole Sesse raccolte dal Dr. E. Bayon. [part]. Annali del Museo Civico di Storia Naturale Giacomo Doria. 56: 93-112.
- Menozzi C. 1942. Formiche dell'isola Fernando Poo e del territorio del Rio Muni (Guinea Spagnola). 24. Beitrag zu den wissenschaftlichen Ergebnissen der Forschungsreise H. Eidmann nach Spanisch-Guinea 1939 bis 1940. Zoologischer Anzeiger 140: 164-182.
- Peeters C., U. Braun, and B. Holldobler. 2013. Large colonies and striking sexual investment in the African stink ant, Paltothyreus tarsatus (subfamily Ponerinae). African Entomology 21(1): 9-14.
- Santschi F. 1910. Formicides nouveaux ou peu connus du Congo français. Annales de la Société Entomologique de France 78: 349-400.
- Santschi F. 1914. Formicides de l'Afrique occidentale et australe du voyage de Mr. le Professeur F. Silvestri. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 8: 309-385.
- Santschi F. 1914. Meddelanden från Göteborgs Musei Zoologiska Afdelning. 3. Fourmis du Natal et du Zoulouland récoltées par le Dr. I. Trägårdh. Göteborgs Kungliga Vetenskaps och Vitterhets Samhälles Handlingar. 15: 1-44.
- Santschi F. 1919. Nouvelles fourmis du Congo Belge du Musée du Congo Belge, à Tervueren. Revue Zoologique Africaine (Brussels) 7: 79-91.
- Santschi F. 1939. Résultats scientifiques des croisières du navire-école belge, "Mercator". XIV. Formicidae s. lt. Mémoires du Musée Royal d'Histoire Naturelle de Belgique. (2)15: 159-167.
- Stephens S. S., P. B. Bosu, and M. R. Wager. 2016. Effect of overstory tree species diversity and composition on ground foraging ants (Hymenoptera: Formicidae) in timber plantations in Ghana. International Journal of Biodiversity Science, Ecosystem Services & management 12(1-2): 96-107.
- Stitz H. 1916. Formiciden. Ergebnisse der Zweiten Deutschen Zentral-Afrika Expedition 1: 369-405.
- Stitz H. 1923. Hymenoptera, VII. Formicidae. Beiträge zur Kenntnis der Land- und Süsswasserfauna Deutsch-Südwestafrikas 2: 143-167.
- Tadu Z., C. Djieto-Lordon, R. Babin, Yede, E. B. Messop-Youbi, and A. Fomena. 2013. Influence of insecticide treatment on ant diversity in tropical agroforestry system: some aspect of the recolonization process. International Journal of Biodiversity and Conservation 5(12): 832-844.
- Taylor B. 1976. Ants of the Nigerian Forest Zone (Hymenoptera: Formicidae). I. Ponerinae, Cerapachyinae, Pseudomyrmecinae. Cocoa Research Institute of Nigeria Technical Bulletin Series 4: 1-41.
- Taylor B., N. Agoinon, A. Sinzogan, A. Adandonon, Y. N'Da Kouagou, S. Bello, R. Wargui, F. Anato, I. Ouagoussounon, H. Houngbo, S. Tchibozo, R. Todjhounde, and J. F. Vayssieres. 2018. Records of ants (Hymenoptera: Formicidae) from the Republic of Benin, with particular reference to the mango farm ecosystem. Journal of Insect Biodiversity 8(1): 006–029.
- Weber N. A. 1943. The ants of the Imatong Mountains, Anglo-Egyptian Sudan. Bulletin of the Museum of Comparative Zoology 93: 263-389.
- Wheeler W. M. 1922. Ants of the American Museum Congo expedition. A contribution to the myrmecology of Africa. II. The ants collected by the American Museum Congo Expedition. Bulletin of the American Museum of Natural History 45: 39-269.
- Wheeler W. M. 1922. Ants of the American Museum Congo expedition. A contribution to the myrmecology of Africa. VIII. A synonymic list of the ants of the Ethiopian region. Bulletin of the American Museum of Natural History 45: 711-1004
- Yeo K., L. M. M. Kouakou, W. Dekoninck, K. Ouattara, and S. Konate. 2016. Detecting intruders: assessment of the anthropophilic ant fauna (Hymenoptera: Formicidae) in the city of Abidjan and along access roads in Banco National Park (Côte d’Ivoire). Journal of Entomology and Zoological Studies 4(4): 351-359.
- Yeo K., T. Delsinne, S. Komate, L. L. Alonso, D. Aidara, and C. Peeters. 2016. Diversity and distribution of ant assemblages above and below ground in a West African forest–savannah mosaic (Lamto, Cote d’Ivoire). Insectes Sociaux DOI 10.1007/s00040-016-0527-6
- Yeo K., and A. Hormenyo. 2007. A Rapid Survey of Ants in Ajenjua Bepo and Mamang River Forest Reserves, Eastern Region of Ghana. Pp 27-29. In McCullough, J., P. Hoke, P. Naskrecki, and Y. Osei-Owusu (eds.). 2008. A Rapid Biological Assessment of the Ajenjua Bepo and Mamang River Forest Reserves, Ghana. RAP Bulletin of Biological Assessment 50. Conservation International, Arlington, VA, USA.
- Pages using DynamicPageList3 parser function
- Photo Gallery
- Need species key
- Tropical
- South subtropical
- Fungus Associate
- Host of Gibellula carnata
- Host of Gibellula liberiana
- Host of Pseudogibellula formicarum
- Host of Sporothrix insectorum
- Host of Verticillium nodulosum
- Host of Akanthomyces gracilis
- Host of Ophiocordyceps sp.
- Host of Polycephalomyces cylindrosporus
- Host of Stilbella buquetti var. formicarum
- Host of Ophiocordyceps australis
- Host of Cordyceps carnata
- Host of Ophiocordyceps myrmecophila
- Species
- Extant species
- Formicidae
- Ponerinae
- Ponerini
- Paltothyreus
- Paltothyreus tarsatus
- Ponerinae species
- Ponerini species
- Paltothyreus species
- Ssr

