Odontomachus simillimus
| Odontomachus simillimus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Odontomachus |
| Species: | O. simillimus |
| Binomial name | |
| Odontomachus simillimus Smith, F., 1858 | |
| Synonyms | |
| |
Found in clearings and secondary growth throughout the Indo-Pacific.
| At a Glance | • Invasive |
Photo Gallery
Identification
Sorger & Zettel (2011) - A member of the Odontomachus haematodus group. Odontomachus simillimus can be easily recognised even in the field by small size, dark colour, proportionally large head and short scape. In the Philippines, there is no other species with a short, truncate subapical tooth of the mandible, and none with fine reticulation on visible part of gaster tergite 2 (but note that the anterior part of tergite 2 which is usually covered by tergite 1 is also reticulate in other species).
Odontomachus simillimus is surprisingly uniform over its large distribution area. It is distinguished from the second Old World species, Odontomachus troglodytes from Africa, Madagascar, and the Seychelles, by its smooth gaster tergite 1.
Satria et al. (2015) - Odontomachus simillimus is easily separated from the other Sumatran species of the genus by the following characteristics of the worker: subapical teeth blunt and short; palp formula 4, 3; pronotal disc and first gastral tergum with several long erect setae. This species is also distinguishable from the other Sumatran species by the following characters of the male: palp formula 6, 3; dorsal outline of clypeus in lateral view much convex; propodeum in lateral view with its dorsal outline angulate; disc of 9th abdominal sternites broader than long, almost as long as apical lobe, with straight basal margin; apical lobe slightly narrower in basal half, with apical margin weakly convex; telomeral apex in lateral view as long as high; vental margin of valviceps with 34–36 denticles; body largely blackish brown, with mandible and small area of clypeus and anterior of head yellowish, and antenna yellowish brown.
Fisher and Smith (2008) - Workers and males are very similar in morphology and size to Odontomachus troglodytes. Bivariate plots of metric measurements did not distinguish the two species. Workers and queen have fine, glossy dorsal striation on head and mesosoma. Metasternal process low and rounded. Metasternal process can be viewed in mounted specimens by removing a hind leg and coxa. Brown (1976) provides a description and references.
Keys including this Species
- Key to Afrotropical Odontomachus species
- Key to Australian Odontomachus Species
- Key to Micronesian Ants
- Key to Odonotomachus of the Indo-Australian Region
- Key to Odontomachus males of Sumatra
- Key to Odontomachus of the Malagasy region
- Key to Odontomachus workers of Sumatra
- Key to Philippine Odontomachus
Distribution
Sorger & Zettel (2011) - Widely distributed from India to Polynesia (Wilson 1959, Brown, 1976), “undoubtedly many of the island records represent accidental introductions by man” (Brown 1976: 87). No distribution limit in the Philippines; records from 21 islands (19 in this study).
Latitudinal Distribution Pattern
Latitudinal Range: 14.63333321° to -27.46667°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Afrotropical Region: Mozambique.
Australasian Region: Australia (type locality), New Caledonia.
Indo-Australian Region: Borneo, Fiji (type locality), Guam, Indonesia (type locality), Kiribati, Krakatau Islands, Malaysia, Marshall Islands, Micronesia (Federated States of), New Guinea, Niue, Northern Mariana Islands, Palau, Philippines, Samoa, Singapore, Solomon Islands, Tonga, Wallis and Futuna Islands.
Oriental Region: Bangladesh, Cambodia, India, Laos, Nicobar Island, Sri Lanka, Thailand, Vietnam.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
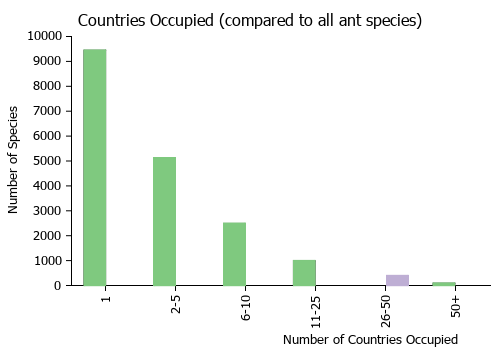
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Habitat
Sorger & Zettel (2011) - Odontomachus simillimus is a common species which also can be found in open or moderately to strongly disturbed habitats, like coastal areas, coconut groves, villages, and even lawns on university campuses. It usually does not enter dense forests, but can be occasionally found on banks of stream running through forests. According to collections by Chapman in eastern Negros, the species can be found from sea level up to an elevation of 900 m (Wheeler and Chapman 1925).
Biology
Satria et al. (2015) - Odontomachus simillimus is a common species in gardens and green patches in residential zones, plantations, and secondary forests. Nests are usually found in the soil near the base of living trees, and under shelters (such as stumps, rotten logs and rocks), but sometimes under paved floors around houses.
In Bali, we found two colonies of O. simillimus near the base of living trees in a cacao plantation which coexisted with colonies of the myrmicine Pheidole ghigii. However, the coexistence between the two species seems to be occasional because we have not yet found such cases in Krakatau and Sumatra. Any other ant partner of O. simillimus has not yet been found.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a associate (details unknown) for the phorid fly Woodiphora pallidinervis (a associate (details unknown)) (Quevillon, 2018).
- This species is a host for the phorid fly Megaselia pagei (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
Flight Period
| X | |||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
 Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Castes
Worker
                   
| |
| . | |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0172668. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
    
| |
| Queen (alate/dealate). Specimen code casent0188531. Photographer Erin Prado, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Male
Images from AntWeb
     
| |
| Male (alate). Specimen code casent0172666. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- simillimus. Odontomachus simillimus Smith, F. 1858b: 80, pl. 5, figs. 8, 9 (q.) FIJI IS, SRI LANKA.
- Type-material: 2 syntype queens.
- Type-localities: Sri Lanka (= Ceylon), and Fiji Is.
- [Note:in BMNH there is a single, damaged queen from Sri Lanka (= Ceylon) which appears to be one of the original type-series, labelled “Ceylon. 50/56.” In addition, BMNH has a single, headless, alate queen from Fiji, without further data, that is also most probably one of the original specimens.]
- Type-depository: BMNH.
- Mayr, 1867a: 79 (w.); Karavaiev, 1925c: 294 (m.); Wheeler, G.C. & Wheeler, J. 1980: 530 (l.); Imai, et al. 1984: 67 (k.); Tjan, Imai, et al. 1986: 57 (k.).
- Junior synonym of haematodus: Roger, 1861a: 24; Roger, 1863b: 22; Mayr, 1863: 437; Mayr, 1865: 64; Mayr, 1872: 148; Emery, 1890b: 44 (footnote, in text); Forel, 1891b: 104; Dalla Torre, 1893: 51; Emery, 1901g: 566; Ruzsky, 1905b: 759; Emery, 1911d: 114; Gallardo, 1918b: 99; Wheeler, W.M. 1919e: 60; Wheeler, W.M. 1922a: 795; Borgmeier, 1923: 78; Kempf, 1972a: 170.
- Status as species: Smith, F. 1859a: 144; Smith, F. 1860a: 72; Smith, F. 1863: 19; Motschoulsky, 1863: 15; Smith, F. 1871a: 319; Chapman & Capco, 1951: 46; Wilson, 1959a: 499; Taylor & Wilson, 1962: 142; Wilson, 1962c: 14; Wilson & Taylor, 1967: 31; Brown, 1976a: 106, 165; Taylor, 1976a: 80; Taylor, 1987a: 49; Radchenko, 1993a: 77; Dlussky, 1994: 53; Bolton, 1995b: 297; Wetterer, 2002: 128; Wetterer & Vargo, 2003: 416; Jaitrong & Nabhitabhata, 2005: 29; Wetterer, 2006: 415; Clouse, 2007b: 266; Framenau & Thomas, 2008: 79; Fisher & Smith, 2008: 15 (redescription); Mohanraj, et al. 2010: 6; Pfeiffer, et al. 2011: 56; Sorger & Zettel, 2011: 158 (redescription); Sarnat & Economo, 2012: 159; Sarnat, et al. 2013: 73; Ramage, 2014: 151; Satria, et al. 2015: 5 (redescription) ; Bharti, Guénard, et al. 2016: 53; Jaitrong, Guénard, et al. 2016: 42.
- Senior synonym of breviceps: Brown, 1976a: 106; Bolton, 1995b: 297; Fisher & Smith, 2008: 15; Satria, et al. 2015: 5.
- Senior synonym of fuscipennis: Wilson, 1959a: 499; Wilson & Taylor, 1967: 31; Brown, 1976a: 106; Bolton, 1995b: 297; Fisher & Smith, 2008: 15; Satria, et al. 2015: 5.
- Senior synonym of pallidicornis: Brown, 1976a: 106; Bolton, 1995b: 297; Fisher & Smith, 2008: 15; Satria, et al. 2015: 5.
- Distribution:
- Malagasy: Seychelles.
- Malesian: Fiji Is, French Polynesia, Indonesia (Ambon, Aru, Bali, Java, Krakatau, Lombok, Nias, Irian Jaya, Seram, Sulawesi, Sumatra, Waigeu), Malaysia (Peninsula, Sabah, Sarawak), Micronesia, New Caledonia, Niue, Papua New Guinea (+ Bismarck Archipelago), Philippines (Balabac, Bantayan, Bayagnan, Busuanga, Camiguin, Catanduanes, Cebu, Dinagat, Hikdop, Leyte, Luzon, Mindanao, Mindoro, Palawan, Samar, Sibuyan, Siquijor, Tablas, Tawi-Tawi), Samoa, Singapore, Solomon Is, Tonga, Vanuatu.
- Oriental: Christmas I., India (+ Andaman Is), Laos, Myanmar, Sri Lanka, Thailand, Vietnam.
- breviceps. Odontomachus haematoda var. breviceps Crawley, 1915b: 239 (w.) CHRISTMAS I.
- Type-material: holotype worker.
- Type-locality: Christmas I.: iii.1914 (D.W. Pinkney).
- Type-depository: BMNH.
- Subspecies of haematodus: Donisthorpe, 1935: 633.
- Junior synonym of simillimus: Brown, 1976a: 106; Bolton, 1995b: 295; Fisher & Smith, 2008: 15; Satria, et al. 2015: 5.
- fuscipennis. Odontomachus haematodes var. fuscipennis Forel, 1913k: 19 (w.q.m.) SRI LANKA, INDONESIA (Sumatra).
- Type-material: syntype workers, syntype queens, syntype males (numbers not stated).
- Type-localities: Sri Lanka (“Ceylon”): Peradeniya (v. Buttel-Reepen), Peradeniya (Bugnion), and Indonesia: Sumatra, Bahsoemboe (v. Buttel-Reepen).
- Type-depository: MHNG.
- Subspecies of haematodus: Forel, 1915a: 22; Santschi, 1919a: 326; Wheeler, W.M. 1924b: 243; Karavaiev, 1925c: 295; Wheeler, W.M. 1934a: 174; Wheeler, W.M. 1935g: 16; Chapman & Capco, 1951: 43.
- Junior synonym of simillimus: Wilson, 1959a: 499; Wilson & Taylor, 1967: 31; Brown, 1976a: 106; Bolton, 1995b: 295; Fisher & Smith, 2008: 15; Satria, et al. 2015: 5.
- pallidicornis. Ponera pallidicornis Smith, F. 1860a: 73 (m.) INDONESIA (Sulawesi).
- Type-material: holotype male.
- Type-locality: Indonesia: Sulawesi, Makassar, “Mak” (A.R. Wallace).
- Type-depository: OXUM.
- Combination in Euponera (Brachyponera): Donisthorpe, 1932c: 458;
- combination in Odontomachus: Brown, 1976a: 106.
- Status as species: Mayr, 1863: 449; Smith, F. 1871a: 322; Dalla Torre, 1893: 40; Emery, 1901g: 566; Emery, 1911d: 116; Donisthorpe, 1932c: 458; Chapman & Capco, 1951: 71.
- Junior synonym of simillimus: Brown, 1976a: 106; Bolton, 1995b: 296; Fisher & Smith, 2008: 15; Satria, et al. 2015: 5.
Type Material
- Odontomachus simillimus: Syntype, queen, Fiji, The Natural History Museum.
- Odontomachus breviceps: Syntype, worker(s), Christmas Island, Australia.
- Ponera pallidicornis: Syntype, male(s), Sulawesi, Indonesia, The Natural History Museum.
The following notes on F. Smith type specimens have been provided by Barry Bolton (details):
Ponera pallidicornis
Holotype male in Oxford University Museum of Natural History. Labelled “Mak” (= Makassar, Sulawesi) and with a Donisthorpe type-label.
Odontomachus simillimus
Type-localities are give as Fiji, and secondarily Ceylon (= Sri Lanka). As far as can be ascertained, all the types were queens. I can find no trace of any Smith material from these localities at Oxford University Museum of Natural History, where the only specimen present in Smith’s collection is a dealate queen from Waigeo I., New Guinea.
In The Natural History Museum there is a single, damaged queen from Ceylon which may be one of the original type-series, labelled “Ceylon. 50/56,” and also with a “Farren White” label. Acc. Reg.: “1850 no. 56 (June 17) Ceylon. Presented by Dr Joseph Hooker FRS.” In addition, The Natural History Museum has a single, headless, alate queen from Fiji, without further data, that may be one of the original specimens.
Determination Clarifications
Known though most of the literature as O. haematode (Linnaeus) 1758, which is a different species (Fisher and Smith 2008).
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Sorger & Zettel (2011) - Worker with smallest HW: CI 83, HL 1.97, HW 1.63, MdI 53, MdL 1.05, MsL 2.43, SI 108, SL 1.77, PnW 0.87, PtH 0.75, PtL 0.64, PtW 0.38, TL 7.63; worker with largest HW: CI 82, HL 2.52, HW 2.07, MdI 53, MdL 1.33, MsL 2.93, PnW 1.13, PtH 0.89, PtL 0.99, PtW 0.52, SI 106, SL 2.20, TL 11.06.
Structures: Mandibles short and stout, with very fine denticles, sometimes completely edentate but always with three apical teeth (intercalary tooth slightly shorter than apical and subapical teeth). Apex of mandibles with some setae. Mandibles mostly smooth, some fine ridges / striae may occur, with fine white pubescence, hair pits distinct. Head in dorsal view rectangular, longer than wide, broadest at level of eyes which do not surpass outline of head. Dorsum of head striate, striation almost reaching nuchal carina (at dorsal margin, area of about the width of the scape, smooth). Eyes located dorsolaterally in first third of head. Mesosoma elongate in dorsal view, broadest at level of pronotum. Pronotum with round striation, often slightly oval or longitudinal in centre, but some entire circles always visible in dorsal view. Mesonotum and propodeum with transverse striation (slightly coarser on propodeum). Mesopleuron smooth in centre, some striation at margins. Metanotal spiracle inconspicuous, situated dorsolaterally. Petiole short and straight, conspicuously “tear-shaped” in frontal view, broad with short petiolar spine, posteriorly flat with transverse striation. Gaster rounded to oval; anterior part of first tergite evenly convex in lateral aspect, without impression; first tergite smooth, second with some reticulation, at least anteriorly.
Pilosity: Fine white semi-appressed pubescence on entire body, very dense on appendages including petiole, on mesosoma, head and gaster distance between hairs approximately their length. Few standing setae on pronotum, several standing hairs on gaster increasing in length towards apex of abdomen. Some isolated hairs on head venter and one pair of standing setae on head dorsum.
Colour: Body, including all appendages, dark brown (almost black in some specimens).
Satria et al. (2015) - (n=10): HW 1.72–2.26 mm, HL 2.12–2.73 mm, SL 1.97–2.43 mm, IFLW 0.50–0.65 mm, EL 0.34–0.42 mm, MDL 1.15–1.50 mm, WL 2.66–3.29 mm, PTL 0.46–0.57 mm, PTH 1.02–1.28 mm, CI 76–83, SI 106–116, MDI 52–56, PTHI 212–236.
Relatively small (HL 2.12–2.73 mm; WL 2.66–3.29 mm). Head in full-face view slightly longer than broad, with posterior margin strongly concave; median furrow on vertex present as dark line; each side of line weakly humped; frontal lobes followed by weak frontal carinae which are divergent posteriad; minimum distance between margin of ocular ridge and margin of compound eye less than half of major axis of compound eye; mandible relatively stout; masticatory margin with small denticles or edentate; subapical tooth shorter than broad, blunt at apex; palp formula 4, 3. Mesosoma in lateral view stout; pronotum including its anteromedian lobe short, in lateral view with anterodorsal slope steep; mesopleuron with conspicuous anteroventral ridge, with anterodorsal margin distinctly carinate, clearly separated by distinct dorsal carina from mesonotum and metapleuron; propodeum in lateral view with dorsum slightly convex and gradually sloping posteriad, with posterior face steeply sloping; propodeal dorsum without median longitudinal depression. Petiolar node conical, with sharply pointed apical spine; node in lateral view, excluding apical spine almost straight anteriorly and very weakly convex posteriorly; apical spine short and slender, 1/4 as long as petiolar height, sometimes weakly curved posteriad; subpetiolar process anteroposteriorly longer than dorsoventrally high, triangular, directed posteriorly. First gastral tergum in lateral view short, with anterior face long and vertical.
Head in full-face view extensively striate, with area between eye and frontal lobe and area around eye smooth and shiny; frontal lobe finely and faintly striate; extraocular furrow striate; median part of vertex along median furrow striate; lateral face weakly striate; venter of head completely or largely smooth and shiny; median disc of clypeus with rough texture. Pronotal disc in dorsal view densely with concentric striation; mesonotum densely striate transversely; mesopleuron largely smooth and shiny, but with anterior third finely striate; metapleuron moderately striate; lateral face of propodeum with transverse striation which is a little sparser and stronger than mesonotum; dorsum and posterior face of propodeum coarsely and transversely striate. Petiolar node weakly striate anteriorly and laterally; posterior face of node weakly striate or sometimes smooth and shiny.
Vertex with a pair of long erect setae; frontal lobe without seta; pronotal disc and first gastral tergum with long erect setae, as long as setae on vertex. Head (except its venter), mesosoma, petiole and gaster with dense subdecumbent to decumbent pubescence; venter of head with sparse appressed pubescence.
Body reddish brown to dark brown (nearly black).
Fisher and Smith (2008) - Measurements: maximum and minimum based on n = 10 from Madagascar: HL 2.33–2.63, HW (across vertex) 1.64–2.03, HW (across upper eye margin) 1.77–2.06, CI 75–81, EL 0.20–0.23, ML 1.14–1.28, MI 48–51, SL 2.16–2.43, SI 109–123, WL 2.62–3.06. FL 2.29–2.56, PW 1.02–1.24.
Queen
Satria et al. (2015) - (n=10): HW 2.07–2.17 mm, HL 2.49–2.69 mm, SL 2.22–2.39 mm, IFLW 0.60–0.68 mm, EL 0.45–0.52 mm, OL 0.08–0.11 mm, MDL 1.31–1.45 mm, WL 3.19–3.29 mm, FWL 6.56–6.86 mm, PTL 0.56–0.60 mm, PTH 1.40–1.48 mm, CI 80–85, SI 102–114, MDI 50–55, PTHI 232–256.
In general appearance queen is similar to worker. Vertex near ocelli swollen; ocular ridge faintly developed; distance between lateral ocelli as long as distance between lateral and median ocelli, and 3.5 times as long as major axis of median ocellus; ocelli in lateral view with not protruded dorsad. Mesosoma with main sclerites associated with wing function, in dorsal view short and stout; anterodorsal slope of pronotum in lateral view relatively steep; anterodorsal outline of mesoscutum in lateral view relatively gentle; mesoscutum without posteromedian depression; parapsidal furrow very weak and slightly curved; mesopleuron without oblique furrow; propodeum relatively long, in lateral view with dorsum almost straight and sloping gradually posteriad. Wing venation as in Figs. 3E and 3F. Petiolar node in lateral view, excluding apical spine with anterior face faintly to weakly concave and posterior face faintly convex; apical spine very short and slender, and curved posteriad; subpetiolar process anteroposteriorly longer than dorsoventrally high. First gastral tergum in lateral view relatively short, with anterior face long and vertical.
Head in full-face view extensively striate, with area between eye and frontal lobe, and area around eye smooth and shiny; frontal lobe finely and faintly striate; extraocular furrow striate; median part of vertex along median furrow striate; lateral face weakly striate; venter of head completely or largely smooth and shiny; median disc of clypeus with rough texture. Pronotum weakly striate transversely; mesoscutum with dense longitudinal striation; striation finer on mesoscutum than on pronotum and propodeum; mesopleuron largely smooth and shiny, but with posteriormost part faintly striate; mesoscutellum smooth and shiny; propodeum strongly striate transversely. Petiolar node excluding apical spine entirely striate, but striation on anterior and posterior faces weaker than that on lateral face.
Pair of long erect setae present on vertex near lateral ocelli; frontal lobe without erect seta; pronotal disc and first gastral tergum with long erect setae, as long as setae on vertex near lateral ocelli. Head, mesosoma, petiole and gaster with dense subdecumbent to decumbent pubescence, except mesopleuron very sparsely pubescent.
Body reddish brown to dark brown (nearly black).
Sorger & Zettel (2011) - Gyne with smallest HW: CI 87, HL 2.27, HW 1.97, MdI 57, MdL 1.30, MsL 2.93, PnW 1.60, PtH 1.00, PtL 0.78, PtW 0.49, SI 107, SL 2.10, TL 9.88; gyne with largest HW: CI 85, HL 2.47, HW 2.10, MdI 54, MdL 1.33, MsL 3.13, PnW 1.65, PtH 1.02, PtL 0.88, PtW 0.61, SI 104, SL 2.18, TL 10.31.
Structures: Differs only in the following characters: pronotum with transverse striation, mesonotum with longitudinal striation, scutellum shiny, sexual female morph-specific characters (wing insertions, mesosoma and gaster bigger).
Fisher and Smith (2008) - Measurements: maximum and minimum based on n = 5 from Madagascar: HL 2.37–2.55, HW (across vertex) 1.79–2.03, HW (across upper eye margin) 1.87–2.13, CI 79–84, EL 0.49–0.53, ML 1.17–1.30, MI 49–52, SL 2.15–2.38, SI 111–118, WL 3.13–3.19. FL 2.36–2.58.
Male
Satria et al. (2015) - (n=10): HW 1.16–1.28 mm, HL 0.95–1.14 mm, SL 0.17–0.21 mm, EL 0.59–0.68 mm, EW 0.34–0.40 mm, OL 0.15–0.18 mm, OES 0.21–0.26 mm, WL 2.66–2.99 mm, FWL 4.60–5.48 mm, PTL 0.46–0.64 mm, PTH 0.81–0.95 mm, CI 104–129, SI 14–17, PTHI 147–176.
Size small (HL 0.95–1.14 mm; WL 2.66–2.99 mm). Major axis of median ocellus smaller than minimum distance between lateral ocelli; antenna 13-merous; scape very short, 1/3 as long as 3rd antennomere; 2nd antennomere 1/2 as long as scape; 3rd to 13th antennomeres each extremely long; palp formula 6, 3; dorsal outline of clypeus in lateral view strongly convex. Mesosoma in lateral view relatively stout and short; dorsal outline of pronotum in lateral view strongly convex; anterodorsal outline of mesoscutum in lateral view steeply slooping; mesoscutum without median depression; parapsidal furrow weak and almost straight; oblique mesopleural furrow relatively deep and wide; ventrolateral part of katepisternum with weak longitudinal furrow; propodeum in lateral view with its dorsal outline angulate; metapleuron distinctly separated from propodeum by a suture; wing venation similar to queen. Petiolar node in lateral view tapering to blunt apex; its anterior slope in lateral view very weakly sinuate, and its posterior slope straight and steeper; subpetiolar process in lateral view anteroposteriorly as long as dorsoventrally high, triangular and thick; petiolar sternum with conspicuously angulate process posteroventraly. First gastral tergum in lateral view short; posterior spine of 8th abdominal tergum long and slender, very weakly curved (but variable in shape within species); pygostyle digitiform, with long setae in apical half; disc of 9th abdominal sternites broader than long, almost as long as apical lobe, with straight basal margin; apical lobe slightly narrower in basal half, with apical margin weakly convex; telomeral apex in lateral view as long as high; distiventral apex of valviceps strongly produced; basiventral corner of valviceps distinctly produced; ventral margin of valviceps with 34–36 denticles.
Head including area between lateral ocelli largely smooth and shiny, with clypeus faintly striate; venter of head faintly striate and shiny. Pronotum largely smooth and shiny, with lateral part faintly striate; mesoscutum faintly rugoso-reticulate and opaque; scuto-scutellar suture with very sparse, weak, longitudinal rugae; mesopleuron with anepisternum smooth and shiny, and katepisternum largely smooth and shiny, but with faint and rough texture in posteriormost part; propodeum including its posterior slope with rough texture. Petiole faintly striate to rugose.
Head, mesosoma, legs, petiole, and gaster with fine dense subdecumbent to decumbent pubescence; apex of mandible, vertex near ocelli, pronotum and gaster with several long erect setae.
Head, mesosoma, legs, petiole, gaster blackish brown; antenna yellowish brown; frons and clypeus largely yellowish brown, with lateral part of clypeus and the areas in front of antennal insertions rather yellow; mandible yellow.
Fisher and Smith (2008) - Measurements: maximum and minimum based on n = 1 from Madagascar: HL 0.89, HW 1.19, CI 133, EL 0.59, SL 0.19, SI 16, WL 2.44. FL 1.73.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- n = 22, 2n = 44, karyotype = 44A (Indonesia) (Imai et al., 1985; Mariano et al., 2015).
- n = 22, 2n = 44, karyotype = 44A (Malaysia) (Goni et al., 1982; Imai et al., 1983; Mariano et al., 2015).
- 2n = 44, karyotype = 44A (Sarawak) (Tjan et al., 1986; Mariano et al., 2015).
References
- Ariani, L., Yulminarti, Herwina, H., Janra, M.N., Satria, R. 2021. Ant community (Hymenoptera: Formicidae) at Ghimbo Potai Traditional Prohibited Forest, Kampar, Riau. IOP Conference Series: Earth and Environmental Science 757, 012080 (doi:10.1088/1755-1315/757/1/012080).
- Barlow, M.M., Bicknell, R.D.C., Andrew, N.R. 2019. Cuticular microstructure of Australian ant mandibles confirms common appendage construction. Acta Zoologica 101, 260–270 (doi:10.1111/azo.12291).
- Brown, W. L., Jr. 1976c. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section A. Introduction, subtribal characters. Genus Odontomachus. Stud. Entomol. 19: 67-171 (page 106, Revived from synonymy, Senior synonym of breviceps and pallidicornis; page 165, see also)
- Dias, R.K.S., Perera, A.P.S. 2016. Species richness of arboreal ant assemblages (Hymenoptera, Formicidae) and frequency of Oecophylla smaragdina (Fabricius) occurrence in a wet zone cashew (Anacardium occidentale L.) field in Sri Lanka. Journal of Science of the University of Kelaniya Sri Lanka 11, 1-10 (doi:10.4038/josuk.v11i1.7996).
- Dias, R.K.S., Rajapaksa, R.P.K.C. 2017. Geographic records of subfamilies, genera and species of ants (Hymenoptera: Formicidae) in the four climatic zones of Sri Lanka: A review. Journal of Science of the University of Kelaniya Sri Lanka 11, 23-45. (doi:10.4038/josuk.v11i2.7999).
- Emery, C. 1911e. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125 (page 114, Junior synonym of haematodus)
- Fisher, B. L. and M. A. Smith. 2008. A Revision of Malagasy Species of Anochetus Mayr and Odontomachus Latreille (Hymenoptera: Formicidae). PloS one. 3:e1787.
- Hasin, S., Tasen, W. 2020. Ant community composition in urban areas of Bangkok, Thailand. Agriculture and Natural Resources 54: 507-514 (doi:10.34044/j.anres.2020.54.5.07).
- Imai, H. T.; Brown, W. L., Jr.; Kubota, M.; Yong, H.-S.; Tho, Y. P. 1984. Chromosome observations on tropical ants from western Malaysia. II. Annu. Rep. Natl. Inst. Genet. Jpn. 34: 66-69 (page 67, karyotype described)
- Ito, F., Hosokawa, R. 2020. Biological notes of Probolomyrmex okinawaensis Terayama & Ogata collected in Yonagunijima Island, and five species of Probolomyrmex collected in Japan and Southeast Asia. Asian Myrmecology 12, e012003 (doi:10.20362/am.012003).
- Karavaiev, V. 1925c. Ponerinen (Fam. Formicidae) aus dem Indo-Australischen Gebiet. (Schluss). Konowia 4: 276-296 (page 294, male described)
- Latumahina, F., Borovanska, M., Musyafa, Sumardi, Susetya Putra, N., Janda, M. 2015. Ants of Ambon Island – diversity survey and checklist. ZooKeys 472, 43–57 (doi:10.3897/zookeys.472.8441).
- Lin, T.-H., Chan, K.-W., Hsu, F.-C., Lin, C.-C., Tseng, H.-Y. 2023. Putative source and niche shift pattern of a new alien ant species (Odontomachus troglodytes) in Taiwan. PeerJ 11, e14718 (doi:10.7717/peerj.14718).
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Mayr, G. 1865. Formicidae. In: Reise der Österreichischen Fregatte "Novara" um die Erde in den Jahren 1857, 1858, 1859. Zoologischer Theil. Bd. II. Abt. 1. Wien: K. Gerold's Sohn, 119 pp. (page 64, Junior synonym of haematodus)
- Mayr, G. 1867a. Adnotationes in monographiam formicidarum Indo-Neerlandicarum. Tijdschr. Entomol. 10: 33-117 (page 79, worker described)
- Mizuno, R., Eguchi, K., Satria, R., Dang, A. V., Bui, V. T., Phung, L. T. H., Ito, F. 2023. Colony composition, phasic reproduction, caste dimorphism, and prey preferences of the oriental non-army doryline ant Yunodorylus eguchii (Borowiec, 2009) (Hymenoptera: Formicidae: Dorylinae). Insectes Sociaux 70(1), 105–117 (doi:10.1007/s00040-023-00898-4).
- Roger, J. 1861a. Die Ponera-artigen Ameisen (Schluss). Berl. Entomol. Z. 5: 1-54 (page 24, Junior synonym of haematodus)
- Satria, R. 2017. Taxonomy of the ant genus Odontomachus (Hymenoptera: Formicidae: Ponerinae) in the Indo-Chinese and Indo-Malayan subregions. Ph.D. thesis, Tokyo Metropolitan University.
- Satria, R., Kurushima, H., Herwina, H., Yamane, S. & Eguchi, K. 2015. The trap-jaw ant genus Odontomachus Latreille from Sumatra, with a new species description. Zootaxa 4048: 1-36.
- Smith, F. 1858b. Catalogue of hymenopterous insects in the collection of the British Museum. Part VI. Formicidae. London: British Museum, 216 pp. (page 80, pl. 5, figs. 8, 9 queen described)
- Sorger, D.M. and H. Zettel. 2011. On the ants (Hymenoptera: Formicidae) of the Philippine Islands: V. The genus Odontomachus LATREILLE, 1804. Myrmecological News. 14:141-163.
- Tjan, K. N.; Imai, H. T.; Kubota, M.; Brown, W. L., Jr.; Gotwald, W. H., Jr.; Yong, H.-S.; Leh, C. 1986. Chromosome observations of Sarawak ants. Annu. Rep. Natl. Inst. Genet. Jpn. 36: 57 (page 57, karyotype described)
- Udayakantha, W.S., Dias, R.K.S., Rajapakse, R.P.K.C. 2023. Geographical records of six common ant species (Hymenoptera: Formicidae) in three climatic zones of Sri Lanka. Caucasian Entomological Bulletin 19(1), 71–78 (doi:10.23885/181433262023191-7178).
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Wheeler, G. C.; Wheeler, J. 1980. Supplementary studies on ant larvae: Ponerinae, Myrmicinae and Formicinae. Trans. Am. Entomol. Soc. 106: 527-545 (page 530, larva described)
- Wilson, E. O. 1959c. Studies on the ant fauna of Melanesia V. The tribe Odontomachini. Bulletin of the Museum of Comparative Zoology 120: 483-510 (page 499, Revived from synonymy, Senior synonym of fuscipennis)
- Yamane, S., Tanaka, H.O., Hasimoto, Y., Ohashi, M., Meleng, P., Itioka, T. 2021. A list of ants from Lambir Hills National Park and its vicinity, with their biological information: Part II. Subfamilies Leptanillinae, Proceratiinae, Amblyoponinae, Ponerinae, Dorylinae, Dolichoderinae, Ectatomminae and Formicinae. Contributions from the Biological Laboratory, Kyoto University 31, 87–157.
- Zettel, H., Sorger, D.M. 2023. Odontomachus yamanei sp.n. (Hymenoptera: Formicidae), a spectacular, previously misidentified ant species from Guadalcanal, Solomon Islands. Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 75: 82–88.
References based on Global Ant Biodiversity Informatics
- Abe T., S. Yamane, and K. Onoyama. Ants collected on the Krakatau Islands 100 years after the great eruptions. Biogeography 14: 65-75.
- Brown Jr., W.L. 1978. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, Tribe Ponerini, Subtribe Odontomachiti, Section B. Genus Anochetus and Bibliography. Studia Entomologia 20(1-4): 549-XXX
- Brown W. L., Jr. 1976. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section A. Introduction, subtribal characters. Genus Odontomachus. Stud. Entomol. 19: 67-171.
- Brown W.L. Jr. 1978. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section B. Genus Anochetus and bibliography. Studia Ent. 20(1-4): 549-638.
- CSIRO Collection
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Clouse R. M. 2007. The ants of Micronesia (Hymenoptera: Formicidae). Micronesica. 39: 171-295.
- Clouse, R.M. 2007. The ants of Micronesia (Hymenoptera: Formicidae), Micronesica 39(2): 171-295.
- Collingwood, C. A. and Van Harten, Antonius. 2001. The Ants (Hym., Formicidae)of Niue, Souh West Pacific. Entomologist's Monthly Magazine. 137:139-143.
- Crawley W. C. 1915. Ants from north and south-west Australia (G. F. Hill, Rowland Turner) and Christmas Island, Straits Settlements. - Part II. Annals and Magazine of Natural History (8)15: 232-239.
- Dias R. K. S. 2006. Current taxonomic status of ants (Hymenoptera: Formicidae) in Sri Lanka. The Fauna of Sri Lanka: 43-52. Bambaradeniya, C.N.B. (Editor), 2006. Fauna of Sri Lanka: Status of Taxonomy, Research and Conservation. The World Conservation Union, Colombo, Sri Lanka & Government of Sri Lanka. viii + 308pp.
- Dias R. K. S. 2013. Diversity and importance of soil-dweeling ants. Proceedings of the National Symposium on Soil Biodiversity, chapt 4, pp 19-22.
- Dias R. K. S., H. P. G. R. C. Ruchirani, K. R. K. A. Kosgamage, and H. A. W. S. Peiris. 2013. Frequency of nest occurrence and nest density of Aneuretus simoni Emery (Sri Lankan Relict Ant) and other ant fauna in an abandoned rubber plantation (Kirikanda Forest) in southwest Sri Lanka. Asian Myrmecology 5: 59-67.
- Dias R. K. S., K. R. K. A. Kosgamage, and H. A. W. S. Peiris. 2012. The Taxonomy and Conservation Status of Ants (Order: Hymenoptera, Family: Formicidae) in Sri Lanka. In: The National Red List 2012 of Sri Lanka; Conservation Status of the Fauna and Flora. Weerakoon, D.K. & S. Wijesundara Eds., Ministry of Environment, Colombo, Sri Lanka. p11-19.
- Dias R. K. S., and R. P. K. C. Rajapaksa. 2016. Geographic records of subfamilies, genera and species of ants (Hymenoptera: Formicidae) in the four climatic zones of Sri Lanka: a review. J. Sci. Univ. Kelaniya 11(2): 23-45.
- Dias R. K. S., and W. S. Udayakantha. 2016. Discovery of the Sri Lankan Relict Ant, Aneuretus simoni Emery (Formicidae, Aneuretinae) and the nest density of the species in a selected region of Meethirigala Forest Reserve, Sri Lanka. Asian Myrmecology 8: 1-8. DOI: 10.20362/am.008005
- Dias R. K. S.; Perera K. A. M. 2011. Worker ant community observed by repeated sampling and information on endemic Aneuretus simoni Emery in the Gilimale Forest Reserve in Sri Lanka. Asian Myrmecology 4: 69-78.
- Dias, R.K.S. 2006. Overview of ant research in Sri Lanka: 2000-2004. ANeT Newsletter 8:7-10
- Dlussky G.M. 1994. Zoogeography of southwestern Oceania. Zhivotnoe naselenie ostrovov Iugo-Zapadnoi Okeanii ekologo-geograficheskie issledovanii 48-93.
- Edwards, John S. and Ian W.B. Thornton. 2001. Colonization of an island volcano, Long Island, Papua New Guinea, and an emergent island, Motmot, in its caldera lake. VI. The pioneer arthropod community of Motmot. Journal of Biogeography. 28. 1379-1388.
- Emery C. 1901. Formiciden von Celebes. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 14:565-580.
- Emery C. 1911. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125.
- Evenhuis N. L., L. G. Eldredge, K. T. Arakaki, D. Oishi, J. N. Garcia, and W. P. Haines. 2010. Terrestrial arthropods surveys on Pagan Island, Northern Marianas. U.S. Fish and Wildlife Service, Pacific Islands Fish & Wildlife Office Honolulu, Hawaii. 72 pages.
- Field Museum Collection, Chicago, Illinois (C. Moreau)
- Fisher B. L., and M. A. Smith. 2008. A revision of Malagasy species of Anochetus Mayr and Odontomachus Latreille (Hymenoptera: Formicidae). PLoS ONE 3(5): e1787. doi:10.1371/journal.pone.0001787
- Forel A. 1913k. Wissenschaftliche Ergebnisse einer Forschungsreise nach Ostindien ausgeführt im Auftrage der Kgl. Preuss. Akademie der Wissenschaften zu Berlin von H. v. Buttel-Reepen. II. Ameisen aus Sumatra, Java, Malacca und Ceylon. Gesammelt von Herrn Prof. Dr. v. Buttel-Reepen in den Jahren 1911-1912. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 36:1-148.
- Forel A. 1915. Fauna Simalurensis. Hymenoptera Aculeata, Fam. Formicidae. Tijdschr. Entomol. 58: 22-43.
- Framenau V.W., and M.L. Thomas. 2008. Ants of Christmas Island (Indian Ocean); identification and distribution. Records of the Western Australian Museum 25: 45-85.
- Greenslade P.J.M. and Greenslade Penelope. 1977. Some Effects of Vegetation Cover and Disturbance on a Tropical Ant Fauna. Insectes Sociaux 24(2): 163-182
- Greenslade P.J.M. and P. Greenslade. 1977. Some effects of vegetation cover and disturbance on a tropical ant fauna. Insectes Sociaux 24(2): 163-182.
- Hashim N.R., W.F.A. Wan Jusoh, and M.N.S. Mohd Nasir. 2010. Ant diversity in a Peninsular Malaysian mangrove forest and oil palm plantation. Asian Myrmecology 3:5-8.
- Hashimoto Y., Y. Morimoto, and M. Mohamed. 2003. Species List of Ground and Leaf Litter Ants Collected in Lower Kinabatangan. Pp 13-18. In Lower Kinabatangan Scientific Expedition 2002, 176 pp. ISBN-13: 983-2369-11-8
- Helms J. A., S. M. Helms, N. I. Fawzi, Tarjudin, F. Xaverius. 2017. Ant community of an Acacia mangium forest in Indonesian Borneo. Serangga 22(1): 147-159.
- Herwina H., and K. Nakamura. 2007. Ant species diversity study using pitfall traps in a small yard in Bogor Botanic garden, West Java, Indonesia. Treubia 35: 99-116.
- Idechill O., R.H. Miller, K.S. Pike, and L.D. Hansen. 2007. Aphids (Hemiptera: Aphididae), ants (Hymenoptera: Formicidae) and associated flora of Palau with comparisons to other Pacific Islands. Micronesica 39(2): 141-171.
- Imai H. T., M. Kubota, W. L. Brown, Jr., M. Ihara, M. Tohari, and R. I. Pranata. 1985. Chromosome observations on tropical ants from Indonesia. Annu. Rep. Natl. Inst. Genet. Jpn. 35: 46-48.
- Imai H. T.; Brown, W. L., Jr.; Kubota, M.; Yong, H.-S.; Tho, Y. P. 1984. Chromosome observations on tropical ants from western Malaysia. II. Annual Report. National Institute of Genetics, Japan 34:66-69.
- Ito, F.; Yamane, S.; Eguchi, K.; Noerdjito, W. A.; Kahono, S.; Tsuji, K.; Ohkawara, K.; Yamauchi, K.; Nishida, T.; Nakamura, K. 2001. Ant species diversity in the Bogor Botanic Garden, West Java, Indonesia, with descriptions of two new species of the genus Leptanilla (Hymenoptera, Formicidae). Tropics 10:379-404.
- Jaitrong W., B. Guenard, E. P. Economo, N. Buddhakala, and S. Yamane. 2016. A checklist of known ant species of Laos (Hymenoptera: Formicidae). Asian Myrmecology 8: 1-32. DOI: 10.20362/am.008019
- Jaitrong W., and T. Ting-Nga. 2005. Ant fauna of Peninsular Botanical Garden (Khao Chong), Trang Province, Southern Thailand (Hymenoptera: Formicidae). The Thailand Natural History Museum Journal 1(2): 137-147.
- Jaitrong W.; Nabhitabhata, J. 2005. A list of known ant species of Thailand. The Thailand Natural History Museum Journal 1(1): 9-54.
- Janda M., G. D. Alpert, M. L. Borowiec, E. P. Economo, P. Klimes, E. Sarnat, and S. O. Shattuck. 2011. Cheklist of ants described and recorded from New Guinea and associated islands. Available on http://www.newguineants.org/. Accessed on 24th Feb. 2011.
- Kami K.S., and S. E. Miller. 1998. Samoan insects and related arthropods: checklist and bibliography. Bishop Museum Technical Report 13, pp 121.
- Kami KS & Miller SE. 1998. Samoan insects and related arthropods: checklist and bibliography. Bishop Museum Technical Report No. 13.
- Karavaiev V. 1925. Ponerinen (Fam. Formicidae) aus dem Indo-Australischen Gebiet. (Schluss). Konowia 4: 276-296.
- Karavaiev V. 1926. Ameisen aus dem Indo-Australischen Gebiet. Treubia 8: 413-445.
- Klimes P., M. Janda, S. Ibalim, J. Kua, and V. Novotny. 2011. Experimental suppression of ants foraging on rainforest vegetation in New Guinea: testing methods for a whole-forest manipulation of insect communities. Ecological Entomology 36: 94-103.
- Klimes P., P. Fibich, C. Idigel, and M. Rimandai. 2015. Disentangling the diversity of arboreal ant communities in tropical forest trees. PLoS ONE 10(2): e0117853. doi:10.1371/journal.pone.0117853
- Latumahina F., M. Borovanska, N. S. Putra, and M. Janda. 2015. Ants of Ambon Island diversity survey and checklist. ZooKeys 472: 4357.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Lucky A., L. E. Alonso, E. Sarnat, and J. Hulr. 2015. Ants and scolytine beetles. In: Richards, S.J. and N. Whitmore (editors) 2015. A rapid biodiversity assessment of Papua New Guinea's Hindenburg Wall region. Wildlife Conservation Society Papua New Guinea Program. Goroka, PNG.
- Mann, W.M. 1919. The ants of the British Solomon Islands. Bulletin of the Museum of Comparative Zoology of Harvard College 63: 273-391
- Mohanraj P., M. Ali, and K. Veerakumari. 2010. Formicidae of the Andaman and Nicobar Islands (Indian Ocean: Bay of Bengal). Journal of Insect Science 10: Article 172
- Mohanraj, P., M. Ali and K. Veenakumari. 2010. Formicidae of the Andaman and Nicobar Islands (Indian Ocean: Bay Of Bengal). Journal of Insect Science 10:172.
- Musthak Ali T. M. 1991. Ant Fauna of Karnataka-1. Newsletter of IUSSI Indian Chapter 5(1-2): 1-8.
- Neville, P. J., O'Dowd, D. J., and Yen, A. L. 2008. Issues and implications for research on disturbed oceanic islands illustrated through an ant survey of the Cocos (Keeling) Islands. J Insect Conserv. 12:313-323.
- Neville, P.J., D.J. O'Dowd and A.L. Yen. 2008. Issues and implications for research on disturbed oceanic islands illustrated through an ant survey of the Cocos (Keeling) Islands. Insect Conserv. 12. 313-323.
- Ogata K. 2005. Asian ant inventory and international networks. Report on Insect inventory Project in Tropic Asia TAIIV: 145-170.
- Overbeck, H. (1924). A List of Ants from Singapore and Neighbouring Places. Journal of the Malayan Branch of the Royal Asiatic Society 2 (1):25-40.
- Pfeiffer M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E. Y.; Kohout, R. J. 2011. The Formicidae of Borneo (Insecta: Hymenoptera): a preliminary species list. Asian Myrmecology 4:9-58
- Philpott S.M., P. Bichier, R.A. Rice, and R. Greenberg. 2008. Biodiversity conservation, yield, and alternative products in coffee agroecosystems in Sumatra, Indonesia. Biodivers. Conserv. 17: 1805-1820. Data obtained from Stacy Philpott
- Radchenko A. G. 1993. Ants from Vietnam in the collection of the Institute of Zoology, PAS, Warsaw. I. Pseudomyrmicinae, Dorylinae, Ponerinae. Annales Zoologici (Warsaw) 44: 75-82.
- Rizali A., A. Rahim, B. Sahari, L.B. Prasetyo, and D. Buchori. 2011. Impact of invasive ant species in shaping ant community structure on small islands in Indonesia. Jurnal Biologi Indonesia 7(2): 221-230.
- Rizali A., Clough Y., Buchori D. and Tscharntke T. 2013. Dissimilarity of ant Communities Increases with Precipitation, but not Reduced Land-Use Intensity, in Indonesian Cacao Agroforestry. Diversity. 5: 26-38
- Rizali A., D. J. Lohman, D. Buchori, L. Budi Prasetyo, H. Triwidodo, M. M. Bos, S. Yamane, and C. H. Schulze. 2009. Ant communities on small tropical islands: effects of island size and isolation are obscured by habitat disturbance and tramp ant species. Journal of Biogeography 37(2): 229-236.
- Rizali A., M. M. Bos, D. Buchori, Sk. Yamane, and C. H. Schulze. 2008. Ants in tropical urban habitats: the myrmecofauna in a densely populated area of Bogor, West Java, Indonesia. Hayati Journal of Biosciences 15(2): 77-84.
- Rizali A., M.M. Bos, D. Buchori, Sk. Yamane, C. Hans, and J. Schulze. 2008. Ants in tropical urban habitats: the myrmecofauna in a densely populated area of Bogor, West Java, Indonesia. Hayati Journal of Biosciences 77-84.
- Rizali A., Y. Clough, D. Buchori, M. L . A. Hosang, M. M. Bos, and T. Tscharntke. 2012. Long-term change of ant community structure in cacao agroforestry landscapes in Indonesia. Insect Conservation and Diversity doi: 10.1111/j.1752-4598.2012.00219.x
- Room P. M. 1975. Diversity and organization of the ground foraging ant faunas of forest, grassland and tree crops in Papua Nez Guinea. Aust. J. Zool. 23: 71-89.
- Room, P.M. 1975. Relative Distributions of Ant Species in Cocoa Plantations in Papua New Guinea Relative Distributions of Ant Species in Cocoa Plantations in Papua New Guinea. Journal of Applied Ecology 12(1):47-61
- Saijo K., and S. Yamane. 2015. Records of ants from the Chuuk State, Micronesia (Hymenoptera, Formicidae). Biogeography 17: 13-15.
- Santschi F. 1919. Cinq notes myrmécologiques. Bulletin de la Société Vaudoise des Sciences Naturelles 52: 325-350.
- Santschi F. 1928. Formicidae (Fourmis). Insects Samoa. 5: 41-58.
- Sarnat Eli M. 2009. The Ants [Hymenoptera: Formicdiae] of Fiji: Systematics, Biogeography and Conservation of an Island Arc Fauna. 80-252
- Satria R., H. Kurushima, H. Herwina, S. Yamane, and K. Eguchi. 2015. The trap-jaw ant genus Odontomachus Latreille (Hymenoptera: Formicidae) from Sumatra, with a new species description. Zootaxa 4048(1): 001-036.
- Savage, A.M., J.A. Rudgers and K.D. Whitney. 2009. Elevated Dominance of Extrafloral Nectary-Bearing Plants Is Associated with Increased Abundances of an Invasive Ant and Reduced Native Ant Richness. Diversity and Distributions 15(5): 751-761
- Smith F. 1863. Catalogue of hymenopterous insects collected by Mr. A. R. Wallace in the islands of Mysol, Ceram, Waigiou, Bouru and Timor. Journal and Proceedings of the Linnean Society of London. Zoology 7: 6-48.
- Snelling R. R. 1998. Insect Part 1: The social Hymenoptera. In Mack A. L. (Ed.) A Biological Assessment of the Lakekamu Basin, Papua New Guinea, RAP 9. 189 ppages
- Snelling R. R. 2000. Ants of the Wapoga river area, Irian Jaya, Indonesia. In Mack, Andrew L. and Leeanne E. Alonso (eds.). 2000. A Biological Assessment of the Wapoga River Area of Northwestern Irian Jaya, Indonesia. RAP Bulletin of Biological Assessment 14, Conservation International, Washington, DC.
- Sorger, D.M. and H. Zettel. 2011. On the ants (Hymenoptera: Formicidae) of the Philippine Islands: V. The genus Odontomachus LATREILLE, 1804. Myrmecological News. 14:141-163.
- Taylor R. W. 1976. The ants of Rennell and Bellona Islands. Natural History of Rennell Island, British Solomon Islands 7: 73-90.
- Terayama M., and S. Haruhiko. 2005. Ants from Guam Island, Mariana islands, Micronesia. Ari 27: 1-5.
- Terayama, M.; Miyano, S.; Kurozumi, T. 1994. Ant fauna (Insecta: Hymenoptera: Formicidae) of the northern Mariana Islands, Micronesia. Natural History Research Special Issue 1:231-236.
- Terayama. M. 2004. Geological and ecological distribution of Japanese ants communities. (translated from Japanese) Reports of the Saitama Prefecture Animal Research Association. 48:27
- Terayama. M. 2004. Geological and ecological distribution of Japanese ants communities. (translated from Japanese) Reports of the Saitama Prefecture Animal Research Association. 48:28
- Tjan K. N., H. T. Imai, M. Kubota, W. L., Jr., Brown, W. H. Gotwald, H.-S. Yong, and C. Leh. 1986. Chromosome observations of Sarawak ants. Annu. Rep. Natl. Inst. Genet. Jpn. 36: 57-58.
- Ward D. 2008. Ecological partitioning and invasive ants (Hymenoptera: Formicidae) in a tropical rain forest ant community from Fiji. Pacific Science 62(4): 473-482.
- Ward, Darren F. and James K. Wetterer. 2006. Checklist of the Ants of Fiji. Fiji Arthropods III 85: 23-47.
- Ward, Darren and Beggs, Jacqueline. 2007. Coexistence, habitat patterns and the assembly of ant communities in the Yasawa islands, Fiji. Ant Oecologica. 32:215-223.
- Watanasit S., J. Saewai, and A. Philapplueng. 2007. Ants of the Klong U-Tapao Basin, Southern Thailand. Asian Myrmecology 1: 69-79.
- Wetterer, James K. 2002. Ants of Tonga. Pacific Science. 56.2: 125-135.
- Wetterer, James K. 2006. Ants (Hymenoptera: Formicidae) of Niue, Polynesia. Pacif Science. 60:(3)413-416.
- Wetterer, James K. and Vargo, Donald Vargo L. 2003. Ants (Hymenoptera: Formicidae) of Samoa. Pacific Science. 57(4):409-419.
- Wheeler, William Morton. 1924. Ants of Krakatau and Other Islands in the Sunda Strait. Treubia. 5(1-3):1-20.
- Wheeler, William Morton.1935.Checklist of the Ants of Oceania.Occasional Papers 11(11): 3-56
- Wilson E. O. 1959. Some ecological characteristics of ants in New Guinea rain forests. Ecology 40: 437-447.
- Wilson E. O. 1959. Studies on the ant fauna of Melanesia V. The tribe Odontomachini. Bulletin of the Museum of Comparative Zoology 120: 483-510.
- Wilson E. O.; Taylor, R. W. 1967. The ants of Polynesia (Hymenoptera: Formicidae). Pacific Insects Monograph 14:1-109.
- Wilson E.O. 1959. Adaptive shift and dispersal in a tropical ant fauna. Evolution 13(1): 122-144.
- Wilson E.O., and G.L. Hunt. 1967. Ant fauna of Futuna and Wallis islands, stepping stones to Polynesia. Pacific Insects 9(4): 563-584.
- Wilson EO & Hunt GL. 1967. Ant fauna of Futuna and Wallis Islands, stepping stones to Polynesia. Pacific Insects 9.4: 563-584.
- Wilson EO, Hunt GL. 1967. Ant fauna of Futuna and Wallis Islands, stepping stones to Polynesia. Pacific Insects 9.4: 563-584.
- Wilson EO, Taylor RW. 1967. Ants of Polynesia. Pacific Insects Monograph 14: 1-109.
- Wilson EO, Taylor RW. 1967. The ants of Polynesia. Pacific Insects Monograph 14:1-109.
- Wilson Edward O. 1959. Adaptive Shift and Dispersal in a Tropical Ant Fauna. Evolution 13(1): 122-144
- Wilson, E.O. XXXX. The ants of the Rennell and Bellona Islands. XXXX 4:13-23
- Wilson, Edward O. 1959. The Ants of Rennell and Bellona Islands. Nat. Hist. Rennell Isl. By Solomon Isl. 4:13-23.
- Wilson, Edward O. and George L. Hunt. 1967. Ant Fauna of Futuna and Wallis Islands, Stepping Stones To Polynesia. Pacific Insects. 9(4):563-584.
- Wilson, Edward O. and Hunt, George L. Jr. 1967. Ant Fauna of Futuna and Wallis Islands, Stepping Stones to Polynesia. Pacific Insects. 9(4):563-584
- Yamane S. 2013. A Review of the ant fauna of the Krakatau Islands, Indonesia. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. Ser: A, 11: 1-66
- Zryanin V. A. 2011. An eco-faunistic review of ants (Hymenoptera: Formicidae). In: Structure and functions of soil communities of a monsoon tropical forest (Cat Tien National Park, southern Vietnam) / A.V. Tiunov (Editor). – M.: KMK Scientific Press. 2011. 277 р.101-124.
- Zryanin V. A., and M. V. Mokrousov. 2015. Contribution to the ant fauna of Lombok Island. Proceedings of the 10th ANeT International Conference, 23-26 October 2015, University of Kelaniya, Sri Lanka. 34
- van Walsum E., B. gobin, F. Ito, and J. Billen. 1998. Worker reproduction in the ponerine ant Odontomachus simillimus. Sociobiology 32: 427-440.
- Pages using DynamicPageList3 parser function
- Invasive
- Photo Gallery
- Tropical
- South subtropical
- Phorid fly Associate
- Host of Woodiphora pallidinervis
- Host of Megaselia pagei
- FlightMonth
- Karyotype
- Species
- Extant species
- Formicidae
- Ponerinae
- Ponerini
- Odontomachus
- Odontomachus simillimus
- Ponerinae species
- Ponerini species
- Odontomachus species
- Ssr









