Neivamyrmex nigrescens
| Neivamyrmex nigrescens | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Dorylinae |
| Genus: | Neivamyrmex |
| Species: | N. nigrescens |
| Binomial name | |
| Neivamyrmex nigrescens (Cresson, 1872) | |
| Synonyms | |
| |
This common species is by far the most widespread species in the United States. As a result of this wide range it is also by far the most studied and best known of the Neivamyrmex.
Photo Gallery
Identification
Smith (1942) – Worker The shape of the mandibles and petiole of the major worker are highly characteristic, as are also the nature of the body sculpturing and color. Also noteworthy are the long scape, the prominent pronotal carina, and the absence of a broad flange in front of the antennal socket. The major worker varies considerably in sculpture and color. The pilosity may also vary in length, but this is often due to wear. The general color ranges from light reddish brown through dark reddish brown into a deep infuscation that approaches black. The sculpturing on the postpetiole is sometimes so delicate as to give this region a slightly shining appearance. The foveolate impressions may vary from a few scattered ones on some specimens to numerous ones on others. Individuals with only a few shallow impressions resemble Neivamyrmex opacithorax; those those with coarser and more numerous impressions approach Neivamyrmex sumichrasti in appearance. There are intergradations in sculpturing between these extremes. The worker is most likely to be confused with that of opacithorax, which it closely resembles in structure and pilosity but from which it differs noticeably in shape of mandibles and nature of sculpturing. The head and usually the postpetiole of nigrescens are heavily sculptured and opaque. The same regions in opacithorax are shining and almost free of conspicuous sculpturing.
Queen There is variation in size of body shape of mandibles, depth and shape of petiolar impressions, length and abundance of hair, color, and amount of sculpture. The mandibles of some specimens are shaped much like those of opacithorax but are broader in porportion to their length. In general, the superior border of the mandible approaches the inferior border toward the apex, making a longer and somewhat more pronounced curve before forming the apical point. The median longitudinal impression on the petiole of most specimens is rather deep, extending the length of the petiole and somewhat widening posteriorly; the Colorado specimen, though, has a very shallow impression. The hair on the body is generally longer and more abundant than that of opacithorax, but this is not always true. The color may range from light ferruginous brown to dark ferruginous brown. The sculpture, though generally similar to that of opacithorax, is usually much coarser, the sculpture on the head being especially rougher.
Male Typical specimens agree very closely with the description, especially in possessing strongly developed frontal carinae; ridges over antennal sockets; rather robust antennae; heavily sculptured body of which the dorsum of the head and thorax is noticeably subopaque owing to the coarser punctation and general nature of the ground surface; blackish head and thorax with slightly lighter gaster; and light-yellowish or grayish pile covering the body. The males are highly variable, different individuals varying with regard to length and robustness of mandibles; depth of groove between frontal carinae, as well as shape of carinae; amount of development of the longitudinal ridge mesad of each eye, and of the ridge above antennal socket; and length of space between lateral ocellus and eye. The thorax may be higher in proportion to its length and possess a weaker transverse prothoracic impression. The sculpturing may be feebler so that parts of the body are less dull, this being especially true of the sides of the thorax. The color of the pilosity may range from light yellowish or grayish to an almost golden yellow; and that of the wings from subhyaline through slightly infuscated to almost blackish.
Identification Keys including this Taxon
Key to the Neivamyrmex species of the United States
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: 40.168817° to 9.06256°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Neotropical Region: Mexico.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Habitat
N. nigrescens shows an extremely wide habitat range. Rarely encountered in desert environments it is nonetheless present, apparently largely confined to canyons and hillsides. Ward (1999) listed the elevational range from sea level to 1460m in California, but we have records up to 2200m in areas outside of California. (Snelling and Snelling 2007)
Biology
Snelling and Snelling (2007) :
Colonies studied by Wheeler (1900) consisted of “thousands” of individuals, while Schneirla (1958) estimated 150,000 to 250,000 workers per nest.
Neivamyrmex nigrescens has a nomadic/statary cycle like Neotropical army ants such as Eciton. The nomadic phase of the cycle begins when pupae eclose to workers. The whole colony then moves along a trail, usually during night hours, capturing any insects they encounter and raiding the nests of other ant species encountered. Columns may be 90m long and are headed by scouts. The colony bivouacs before dawn, using natural cavities or nests of other species, which they have pillaged. The following night they again move and raid. This nomadic cycle lasts for about three weeks or until the larvae in the colony (which they transport each night) begin to pupate. The statary phase then begins and the ants nest in subterranean cavities, either under stones or in abandoned ant nests for about 18 days (Schneirla, 1958). Raids continue but are less extensive than during the nomadic phase.
New colonies of N. nigrescens are formed when “a daughter queen leaves the parental nest, accompanied by a number of workers. A mature colony is capable of producing a small number of females, some of which may be fertilized in the nest by their brothers, but this does not preclude mating outside the nest, or with males of other colonies. Since females are never winged, they can make no nuptial flight.” (Smith 1965). Recent very preliminary data for this species suggest that N. nigrescens may, at least at times, have more than one functional queen present in the colony.(D. Kronauer, pers. comm.)
Other ants form an important part of the diet of N. nigrescens. Mallis (1938) observed this species carrying larvae and pupae of Tetramorium caespitum (Linné), as well as click beetles, mayflies, water boatmen and crickets. Wheeler & Long (1901) found larvae of Solenopsis geminata (Fabr.) and three species of Pheidole, as well as dead carabid beetles, in nests they studied in Texas. Ward (1999) further notes that in California Veromessor andrei (Mayr), Pheidole californica Mayr, Pheidole hyatti Emery, Solenopsis molesta (Say) and Formica moki Wheeler are also prey items of this species. In Arizona N. nigrescens has been observed regularly raiding Pheidole obtusospinosa Pergande (as P. subdentata) and Pheidole desertorum Wheeler. Neece & Bartell (1982) noted the presence of unidentified mites of the family Trachyaropodidae in colonies of N. nigrescens.
The blind snake, Leptotyphlops dulcis, is able to follow the pheromone trails of N. nigrescens to locate columns and feed on the ant brood (Watkins et al., 1967). When the army ants attack the snake it forms a protective ball-like coil and smears a cloacal fluid on its body, which discourages further ant attacks (Watkins et al., 1972).
Several species of scuttle flies (Diptera: Phoridae) are known to parasitize adults of N. nigrescens. These include species in the genera Dacnophora and Cremersia (B. V. Brown, pers. comm.), and Xanionotum (Rettenmeyer and Akre 1968). The diapriid wasp, Ecitovagus gibbus Masner has been found as a parasitoid of N. nigrescens in southeastern Arizona (Masner 1977). Myrmecophilous Staphylinidae (Coleoptera) associated with this ant in areas other than California include: Microdonia laticollis Brues, M. nitidiventris Brues, M. occipitalis Casey, Ecitoxenidia brevicornis Seevers, E. brevipes Brues, Dinocoryna carolinensis Seevers, and Ecitonidia wheeleri Wasmann (Seevers 1965). At least two species in the carabid beetle genus Helluomorphoides (H. ferrugineus Casey and H. latitarsis LeConte) are specialized predators on both the booty and brood of N. nigrescens in southeastern Arizona: “The beetles were observed running in army ant columns or standing off to the sides of the columns, behind rocks or beneath clusters of leaf litter. During their predatory activities, beetles ran along the trails in both directions, 'plowing' through the continuous two-way ant traffic. When a beetle of either species contacted a worker ant bringing booty back to her bivouac, the ant usually dropped the booty. On some occasions, if the booty was a larval or pupal individual of another ant species, the beetle immediately ate it and continued on the trail. On other occasions the beetle picked up the dropped booty, left the raiding column, and proceeded to a nearby rock. There, the beetle quickly ate the larva or pupa, returned to the column, and resumed running along the trail” (Topoff, 1969). Beetles were observed to forcibly take booty from the ants. The beetles were also seen to feed on brood caches of the Neivamyrmex colony with which they became associated.
For further information on the biology and behavior of this species, see Ward (1999).
Wheeler (1903) - A fine colony of Eciton schmitti which I kept a few years ago, exhibited this restlessness in a striking and ludicrous manner. The colony at first confined in a tall glass jar on a square board surrounded by a water moat. The ants kept going up and down the inside of the jar in files for many hours. Finally removed the lid. The file at advanced over the rim and descended on the outer surface till it reached the circular base of the jar where it turned to the left at a right angle and proceeded completely around the base till it met the column at the turning point. To my surprise it kept right on over the same circumference which was long enough to accommodate the whole colony. The ants continued going round and round the circular base of the jar, following one another like so many sheep, without the slightest inkling that they were perpetually traversing the same path. They behaved exactly as they do on one of their predatory expeditions. They kept up this gyration for 46 hours before the column broke and spread over the board to the water’s edge and clustered in the manner so characteristic of this and the allied species (E. opacithorax, sumicrasti, etc.). I have never seen a more astonishing exhibition of the limitations germanice "Bornirtheit" of instinct. For nearly two whole days these blind creatures, so dependent on the contact-odor sense of their antennae, kept palpitating their uniformly smooth, odoriferous trail and the advancing bodies of the ants immediately preceding them, without perceiving that they were making no progress but only wasting their energy, till the spell was finally broken by some more venturesome members of the colony. Recently I have found a remarkable observation of the same kind recorded by Fabre in the 6th volume of his incomparable “Souvenirs Entomologiques.” He describes an army of caterpillars of the “processionnaire du pin” (Cnethocampa pityocampa) going round and round the outside of a large vase x.35 m. in circumference for seven days! During this period the caterpillars were on the march 84 hours altogether, stopping to rest on their path only when overtaken by the cold, and not actually deviating till the eighth day. Fabre estimated that the caterpillars crawled around the vase 335 times! In this case the insects were not guided by contact-odor like the Ecitons, but by the silken thread spun by each individual over the surface traversed.
In the mountains of southern Arizona, two army ant species, Neivamyrmex nigrescens and Neivamyrmex rugulosus, prey on Trachymyrmex arizonensis (Miranda et al. 1980, LaPolla et al. 2002). In Tamaulipas, Mexico, Neivamyrmex texanus was observed raiding a colony of Trachymyrmex saussurei (Rabeling & Sanchez-Peña, unpublished data). Based on these few observations, army ants seem to be important predators of at least some Trachymyrmex species, and their raids may result in a significant brood loss and partial destruction of the fungus garden (LaPolla et al. 2002).
Brown & Fenner (1998) report this species conducting raids on the nests of Pheidole obtusospinosa at Pefia Blanca Lake, AZ, USA.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a host for the phorid fly Dacnophora pectinatus (a parasite) in Arizona, USA (Brown & Fenner, 1998).
- This species is a host for the phorid fly Cremersia sp. (a parasite) in Arizona, USA (Brown & Fenner, 1998).
- This species is a associate (details unknown) for the phorid fly Ecitomyia wheeleri (a associate (details unknown)) (Quevillon, 2018).
- This species is a host for the diapriid wasp Ecitovagus gibbus (a parasite) (www.diapriid.org) (potential host).
- This species is a associate (details unknown) for the phorid fly Ecitomyia wheeleri (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the phorid fly Xanionotum hystrix (a associate (details unknown)) (Quevillon, 2018).
- This species is a associate (details unknown) for the phorid fly Xanionotum wasmanni (a associate (details unknown)) (Quevillon, 2018).
Flight Period
| X | |||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
 Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Life History Traits
- Queen number: monogynous (Holiday, 1904; Schneirla, 1958; Rissing and Pollock, 1988; Frumhoff & Ward, 1992; Mizuno et al., 2021)
- Queen type: dichthadiiform (Holiday, 1904; Schneirla, 1958; Mizuno et al., 2021)
- Mean colony size: 30,000-140,000 (Holiday, 1904; Schneirla, 1958; Topoff et al., 1980; Beckers et al., 1989; Mizuno et al., 2021)
- Foraging behaviour: group hunter (Topoff et al., 1980; Beckers et al., 1989)
Castes
Worker
   
| |
| . | |
Images from AntWeb
   
| |
| Worker. Specimen code casent0102766. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code casent0104124. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Queen
Images from AntWeb
   
| |
| Queen (ergatoid). Specimen code casent0104061. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Male
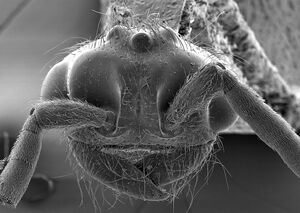     
| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- nigrescens. Labidus nigrescens Cresson, 1872: 194 (m.) U.S.A. (Texas).
- Type-material: holotype male.
- Type-locality: U.S.A.: Texas, Bosque County (G.W. Belfrage).
- Type-depository: ANSP.
- Wheeler, W.M. 1900a: 563 (q.); Smith, M.R. 1942c: 551 (w.); Wheeler, G.C. & Wheeler, J. 1984: 273 (l.).
- Combination in Eciton: Dalla Torre, 1893: 5;
- combination in E. (Labidus): Emery, 1895c: 261;
- combination in E. (Acamatus): Emery, 1900a: 187;
- combination in E. (Neivamyrmex): Smith, M.R. 1942c: 550; Gregg, 1963: 286;
- combination in Neivamyrmex: Borgmeier, 1955: 494.
- Status as species: Cresson, 1887: 259; Dalla Torre, 1893: 5; Emery, 1895c: 261, Emery, 1900a: 179; Wheeler, W.M. 1908e: 417 (redescription); Emery, 1910b: 27; Wheeler, W.M. 1910g: 562; Smith, M.R. 1938b: 160; Smith, M.R. 1942c: 550 (redescription); Buren, 1944a: 280; Creighton, 1950a: 73; Smith, M.R. 1951a: 781; Cole, 1953c: 84; Borgmeier, 1955: 494 (redescription); Smith, M.R. 1958c: 109; Smith, M.R. 1967: 345; Watkins, 1971: 94 (in key); Kempf, 1972a: 157; Watkins, 1972: 358; Hunt & Snelling, 1975: 21; Watkins, 1976: 15 (in key); Smith, D.R. 1979: 1331; Snelling, R.R. & George, 1979: 31; Petralia & Vinson, 1980: 378; Dlussky, 1981a: 48; Watkins, 1982: 212 (in key); Watkins, 1985: 482 (in key); Wheeler, G.C. & Wheeler, J. 1986g: 19 (in key); DuBois & LaBerge, 1988: 136; Mackay, Lowrie, et al. 1988: 87; Bolton, 1995b: 290; Ward, 1999a: 84 (redescription); Mackay & Mackay, 2002: 61; Coovert, 2005: 32; MacGown & Forster, 2005: 67; Ward, 2005: 62; Snelling, G.C. & Snelling, 2007: 484; Deyrup, 2017: 37.
- Senior synonym of schmitti: Smith, M.R. 1938b: 160; Smith, M.R. 1942c: 550; Buren, 1944a: 280; Creighton, 1950a: 73; Smith, M.R. 1951a: 781; Borgmeier, 1953: 6; Borgmeier, 1955: 494; Kempf, 1972a: 157; Smith, D.R. 1979: 1331; Snelling, R.R. & George, 1979: 31; Bolton, 1995b: 290; Ward, 1999a: 84; Coovert, 2005: 32; Snelling, G.C. & Snelling, 2007: 485.
- Distribution: Mexico, U.S.A.
- schmitti. Eciton (Acamatus) schmitti Emery, 1894c: 183 (diagnosis in key) (w.) U.S.A. (Missouri).
- Type-material: syntype workers (number not stated).
- Type-locality: U.S.A.: Missouri, Ripley County, Doniphan (Pergande?).
- Type-depository: MSNG.
- Emery, 1895c: 258 (w.); Wheeler, W.M. & Long, 1901: 161 (m.); Wheeler, G.C. 1943: 331 (l.).
- Status as species: Emery, 1895c: 258 (description), Pergande, 1896: 874; Forel, 1899c: 28; Wheeler, W.M. & Long, 1901: 161; Wheeler, W.M. 1904e: 300; Wheeler, W.M. 1908e: 410; Emery, 1910b: 25; Wheeler, W.M. 1910g: 562; Essig, 1926: 869; Smith, M.R. 1930a: 2; Wheeler, W.M. 1932a: 2; Borgmeier, 1936: 59; Dennis, 1938: 278.
- Junior synonym of nigrescens: Smith, M.R. 1938b: 160; Smith, M.R. 1942c: 550; Buren, 1944a: 280; Creighton, 1950a: 73; Smith, M.R. 1951a: 781; Borgmeier, 1953: 6; Borgmeier, 1955: 494; Kempf, 1972a: 157; Smith, D.R. 1979: 1331; Snelling, R.R. & George, 1979: 31; Bolton, 1995b: 291; Ward, 1999a: 84; Coovert, 2005: 32; Snelling, G.C. & Snelling, 2007: 485.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Smith (1942) - Major. Length 4-5 mm.
Head scarcely longer than broad, narrowed posteriorly; posterior border emarginate, forming very distinctly produced, sharp, angular corners which are often somewhat outwardly curved but not so pronouncedly as with wheeleri. Eye ocellus-like, convex, usually very distinct because of the opaque appearance given head by sculpturing. Mandible with basal tooth on superior border lacking or very faintly indicated; margin between where his tooth should be and masticatory border convex, instead of straight or excised as in some of the other species; discal area of exterior surface flattened. Scape approximately three and one-half times as long as wide, extending beyond posterior border of eye a distance almost equivalent to greatest width of scape; funiculus with segments 2 to 4 inclusive at most scarcely broader than long. Frontal carina not forming a broad, distinct flange in front of antennal socket as in wheeleri. Dorsum of thorax, from above, less convex laterally than in wheeleri, thus giving thorax a more compressed appearance. Side of prothorax extending above fore coxae as a prominent, somewhat reflexed lobe. Promesonotum, in profile, appearing as an arch which merges into basal surface of epinotum without forming as abrupt an angular termination as in some species. Basal surface of epinotum meeting declivity in a rather rounded, obtuse angle. Petiole, from above, approximately two-thirds as broad as long; anteroventral surface of peduncle with bluntly rounded tooth. Postpetiole, from above, subtrapezoidal, slightly shorter than petiole, approximately as long as broad; almost anterior half somewhat laterally margined on each side.
Head, thorax, petiole, and postpetiole opaque, covered with dense granulate punctures, interspersed with coarse foveolate impressions; head and thorax most heavily sculptured, petiole and postpetiole least of all; legs subopaque or faintly shining, gaster smooth and shining. Discal surface of mandible bearing fine rugulae and coarse, scattered punctures which give an opaque appearance; borders of mandible more shining.
Hairs yellowish, rather abundarit, of various lengths, suberect to erect, many unusually long.
Head, thorax, petiole, and postpetiole usually deep reddish brown, sometimes almost blackish; gaster and legs slightly lighter. Eye yellow or amber.
Queen
Smith (1942) - Length 10-14 mm.
Head approximately as Ions as broad, broadest anteriorly; posterior border emarginate. Eye ocelluslike, rather large, much larger and more distinct than that of opacithorax. Mandible of somewhat similar shape to that of opacithorax but usually more robust. Scape curved, rather robust, approximately one-half length of head. Region adjacent to and also somewhat posterior to frontal area rather angularly produced anteriorly. A conspicuous median groove extending posteriorly from clypeus toward vertex, becoming feebler posteriorly. Dorsal surface of clypeus concave, middle of anterior border broadly but shallowly excised. Dorsal surface of head with deep median impression near occipital border and a groove leading from this toward front of head, also with a distinct impression on each side of head in front of posterior corners; these impressions giving back of head an extended effect and causing posterior corners to have an unusually angulate or tuberculate appearance. Thorax, from above, more than twice as long as wide, gradually increasing in width posteriorly to metanotum; epinotum not so wide as head. Dorsal thoracic sutures distinct. Pronotum approximately as broad as long, marginate anteriorly and laterally. Mesonotum with a somewhat angular anterior border, and a more broadly angular posterior border. Epinotum broader than long, with bluntly angular posterior corners. Mesonotum and epinotum with conspicuous longitudinal, median impression. Petiole, in profile, of approximately same height as epinotum but not so long; peduncle with large, convex protuberance beneath; from above, not one and a half times as broad as long, scarcely broader in front than behind, and with somewhat subparallel sides, and a deep median, longitudinal impression which widens posteriorly.
Head and thorax opaque, owing to the dense 'granulate shagreening and the scattered, deep punctures. Petiole more finely sculptured than head or thorax.
Hairs fairly abundant on head, thorax, petiole, and appendages; clypeus, gula, mandibles, and sea pes with longer hairs of variable length.
Light or deep ferruginous brown.
Male
Smith (1942) - Length 11.25-13 mm.
Head approximately one and eight-tenths times as broad as long; posterior border rounded. Eye rather small, moderately convex, and protuberant. Ocelli very small, placed on low protuberance which is only slightly elevated above general surface of head; summit of protuberance concave; lateral ocellus far removed from eye, this space often greater than the space between the two lateral ocelli. Frontal carinae converging behind, with distinct but somewhat feeble groove between them leading to anterior ocellus. Ridge over antennal socket remarkably well developed, forming a large, thick welt which tends partly to obscure the posterior border of the head, when the head is viewed anteriorly. Antenna distinctly more robust than that of opacithorax; scape slightly shorter than combined length of first 3 funicular segments; segments 2 to 6 inclusive much more bro:!dened than those of opacithorax, all segments except first clearly longer than broad. Mandible moderately elongate to rather elongate, with subparallel superior and inferior borders basally, superior border converging with inferior border somewhere between apical half to third of mandible and forming a rather blunt point. Head, from above, with very prominent frontal carinae and a very strong, protuberant ridge above each antennal socket; a transverse groove behind each ridge. Head not noticeably extended behind eyes, posterior corners well rounded, the curvature blending into that of eyes. Eye, in profile, narrowed above, not occupying all of side of head, there being an area mesad and ventrad of it larger than similar areas of opacithorax, and a much larger area posterodorsad than the two areas mentioned; head behind ocelli convex, without occipital flange. Thorax, in profile, approximately one and one-half times as long as high, not extended anteriorly above head. Prothorax anteriorly with a distinct, transverse impression. Epinotum truncate in appearance but really weakly concave. Mesonotum with distinct anteromedian and parapsidal lines. Tarsal claws feebly toothed. Gaster rather robust, more noticeably so than in opacithorax; with pronounced constrictions between segments, and a transverse impression near base of sixth gastric tergum. Intermediate tooth of seventh gastric sternum small, often not clearly seen.
Body more opaque than that of opacithorax, especially on dorsum of head and thorax, where the coarse punctation and general ground surface of these regions obscure the shining effect. Gaster also more coarsely sculptured than that of opacithorax.
Hairs light yellowish or grayish to deeper yellow, sometimes almost golden; rather closely appressed on all parts of body except antennal scapes, head, and ventral surfaces of thorax and petiole, where they are longer and more nearly erect.
Head and thorax almost black; gaster lighter brown; funiculi and tarsi usually lighter. Wings ranging from subhyaline through slightly infuscated to deeply infuscated; veins and stigma brownish to blackish.
Type Material
Smith (1942) - Texas, G. W. Belfrage. Holotype in Academy of Natural Sciences of Philadelphia.
References
- Alatorre-Bracamontes, C.E., Vásquez-Bolaños, M. 2010. Lista comentada de las hormigas (Hymenoptera: Formicidae) del norte de México. Dugesiana 17(1): 9-36.
- Beckers R., Goss, S., Deneubourg, J.L., Pasteels, J.M. 1989. Colony size, communication and ant foraging Strategy. Psyche 96: 239-256 (doi:10.1155/1989/94279).
- Borgmeier, T. 1955. Die Wanderameisen der neotropischen Region. Stud. Entomol. 3: 1-720 (page 494, Combination in Neivamyrmex, Senior synonym of schmitti)
- Boudinot, B. E., Moosdorf, O. T. D., Beutel, R. G., Richter, A. 2021. Anatomy and evolution of the head of Dorylus helvolus (Formicidae: Dorylinae): Patterns of sex‐ and caste‐limited traits in the sausagefly and the driver ant. Journal of Morphology 282(11), 1616–1658 (doi:10.1002/jmor.21410).
- Brown, B.V., Fenner, D.H. 1998. Parasitic phorid flies (Diptera: Phoridae) associated with army ants (Hymenoptera: Formicidae: Ecitoninae, Dorylinae) and their conservation biology. Biotropica 30: 482-487.
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Cresson, E. T. 1872. Hymenoptera Texana. Trans. Am. Entomol. Soc. 4: 153-292 (page 194, male described)
- Dalla Torre, K. W. von. 1893. Catalogus Hymenopterorum hucusque descriptorum systematicus et synonymicus. Vol. 7. Formicidae (Heterogyna). Leipzig: W. Engelmann, 289 pp. (page 5, Combination in Eciton)
- Emery, C. 1895d. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. (Schluss). Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 8: 257-360 (page 261, Combination in E. (Labidus))
- Emery, C. 1900e. Nuovi studi sul genere Eciton. Mem. R. Accad. Sci. Ist. Bologna (5)8:173-188 (page 187, Combination in E. (Acamatus))
- Hill, J.G. 2015. Ants (Hymenoptera: Formicidae) of the Big Thicket Region of Texas. Midsouth Entomologist 8: 24-34.
- MacGown, J.A., Booher, D., Richter, H., Wetterer, J.K., Hill, J.G. 2021. An updated list of ants of Alabama (Hymenoptera: Formicidae) with new state records. Transactions of the American Entomological Society 147: 961-981 (doi:10.3157/061.147.0409).
- Mizuno, R., Suttiprapan, P., Jaitrong, W., Yamada, A., Ito, F. 2021. Colony composition, phasic reproduction, and queen–worker dimorphism of an oriental non-army ant doryline Cerapachys sulcinodis species complex in northern Thailand. Insectes Sociaux (doi:10.1007/s00040-021-00841-5).
- Snelling, G. C.; Snelling, R. R. 2007. New synonymy, new species, new keys to Neivamyrmex army ants of the United States. In Snelling, R. R., B. L. Fisher, and P. S. Ward (eds). Advances in ant systematics (Hymenoptera: Formicidae): homage to E. O. Wilson - 50 years of contributions. Memoirs of the American Entomological Institute 80:459-550. PDF
- Petralia, R. S.; Vinson, S. B. 1980 [1979]. Comparative anatomy of the ventral region of ant larvae, and its relation to feeding behavior. Psyche (Camb.) 86: 375-394 (page 378, see also)
- Smith, M. R. 1938b. Notes on the legionary ants (Eciton, subgenus Acamatus) with a record of new specific synonymy. Proc. Entomol. Soc. Wash. 40: 157-160 (page 160, Senior synonym of schmitti)
- Smith, M. R. 1942c. The legionary ants of the United States belonging to Eciton subgenus Neivamyrmex Borgmeier. Am. Midl. Nat. 27: 537-590 (page 551, male described; page 550, Combination in E. (Neivamyrmex))
- Topoff, H.R. 1982. Behavioral ecology of the army ant Neivamyrmex nigrescens in a desert-grassland habitat. Pp. 98-102 in The biology of social insects, M. Breed, C.D. Michener, and H.E.Evans, eds. Westview Press, Boulder.
- Varela-Hernández, F., Medel-Zosayas, B., Martínez-Luque, E.O., Jones, R.W., De la Mora, A. 2020. Biodiversity in central Mexico: Assessment of ants in a convergent region. Southwestern Entomologist 454: 673-686.
- Ward, P. S. 1999a. Deceptive similarity in army ants of the genus Neivamyrmex (Hymenoptera: Formicidae): taxonomy, distribution and biology of N. californicus (Mayr) and N. nigrescens (Cresson). J. Hym. Res. 8: 74-97 (page 84, see also)
- Watkins, J. F., II. 1972. The taxonomy of Neivamyrmex texanus, n. sp., N. nigrescens and N. californicus (Formicidae: Dorylinae), with distribution map and keys to the species of Neivamyrmex of the United States. J. Kans. Entomol. Soc. 45: 347-372 (page 358, see also)
- Wheeler, G. C.; Wheeler, J. 1984a. The larvae of the army ants (Hymenoptera: Formicidae): a revision. J. Kans. Entomol. Soc. 57: 263-275 (page 273, larva described)
- Wheeler, W. M. 1900a. The female of Eciton sumichrasti Norton, with some notes on the habits of Texan Ecitons. Am. Nat. 34: 563-574 (page 563, queen described)
References based on Global Ant Biodiversity Informatics
- Bestelmeyer B. T., and J. A. Wiens. 2001. Local and regional-scale responses of ant diversity to a semiarid biome transition. Ecography 24: 381-392.
- Borgmeier T. 1936. Sobre algumas formigas dos generos Eciton e Cheliomyrmex (Hym. Formicidae). Archivos do Instituto de Biologia Vegetal (Rio de Janeiro) 3: 51-68.
- Borgmeier T. 1953. Vorarbeiten zu einer Revision der neotropischen Wanderameisen. Studia Entomologica 2: 1-51.
- Borgmeier T. 1955. Die Wanderameisen der neotropischen Region. Studia Entomologica 3: 1-720.
- Borgmeier T. 1957. Die Maxillar- und Labialtaster der neotropischen Dorylinen (Hym. Formicidae). Revista Brasileira de Biologia 17: 387-394.
- Boulton A. M., Davies K. F. and Ward P. S. 2005. Species richness, abundance, and composition of ground-dwelling ants in northern California grasslands: role of plants, soil, and grazing. Environmental Entomology 34: 96-104
- Buren W. F. 1941. A preliminary list of Iowa ants. Iowa State College Journal of Science 15: 111-117
- Carroll T. M. 2011. The ants of Indiana (Hymenoptera: Formicidae). Master's Thesis Purdue university, 385 pages.
- Cokendolpher J. C., and O. F. Francke. 1990. The ants (Hymenoptera, Formicidae) of western Texas. Part II. Subfamilies Ecitoninae, Ponerinae, Pseudomyrmecinae, Dolichoderinae, and Formicinae. Special Publications, the Museum. Texas Tech University 30:1-76.
- Cole A. C. 1940. A Guide to the Ants of the Great Smoky Mountains National Park, Tennessee. American Midland Naturalist 24(1): 1-88.
- Coovert, G.A. 2005. The Ants of Ohio (Hymenoptera: Formicidae) Ohio Biological Survey Bulletin New Series Volume 15(2):1-196
- Cover S. P., and R. A. Johnson. 20011. Checklist of Arizona Ants. Downloaded on January 7th at http://www.asu.edu/clas/sirgtools/AZants-2011%20updatev2.pdf
- Dash S. T. and L. M. Hooper-Bui. 2008. Species diversity of ants (Hymenoptera: Formicidae) in Louisiana. Conservation Biology and Biodiversity. 101: 1056-1066
- Dattilo W. et al. 2019. MEXICO ANTS: incidence and abundance along the Nearctic-Neotropical interface. Ecology https://doi.org/10.1002/ecy.2944
- Des Lauriers J., and D. Ikeda. 2017. The ants (Hymenoptera: Formicidae) of the San Gabriel Mountains of Southern California, USA with an annotated list. In: Reynolds R. E. (Ed.) Desert Studies Symposium. California State University Desert Studies Consortium, 342 pp. Pages 264-277.
- Deyrup M. 2016. Ants of Florida: identification and natural history. CRC Press, 423 pages.
- DuBois M. B. 1981. New records of ants in Kansas, III. State Biological Survey of Kansas. Technical Publications 10: 32-44
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-295
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-296
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-297
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-298
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-299
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-300
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-301
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-302
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-303
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-304
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-305
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-306
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-307
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-308
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-309
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-310
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-311
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-312
- DuBois M. B. 1985. Distribution of ants in Kansas: subfamilies Ponerinae, Ecitoninae, and Myrmicinae (Hymenoptera: Formicidae). Sociobiology 11: 153-315
- DuBois M. B. 1988. Distribution of army ants (Hymenoptera: Formicidae) in Illinois. Entomological News 99: 157-160.
- Dubois, M.B. and W.E. Laberge. 1988. An Annotated list of the ants of Illionois. pages 133-156 in Advances in Myrmecology, J. Trager
- Eastlake Chew A. and Chew R. M. 1980. Body size as a determinant of small-scale distributions of ants in evergreen woodland southeastern Arizona. Insectes Sociaux 27: 189-202
- Emery C. 1895. Beiträge zur Kenntniss der nordamerikanischen Ameisenfauna. (Schluss). Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 8: 257-360.
- Emery C. 1910. Hymenoptera. Fam. Formicidae. Subfam. Dorylinae. Genera Insectorum 102: 1-34.
- Fernandes, P.R. XXXX. Los hormigas del suelo en Mexico: Diversidad, distribucion e importancia (Hymenoptera: Formicidae).
- Forster J.A. 2005. The Ants (hymenoptera: Formicidae) of Alabama. Master of Science, Auburn University. 242 pages.
- Gibbs M. M., P. L. Lambdin, J. F. Grant, and A. M. Saxton. 2003. Ground-inhabiting ants collected in a mixed hardwood southern Appalachian forest in Eastern Tennessee. Journal of the Tennessee Academy of Science 78(2): 45-49.
- Gregg, R.T. 1963. The Ants of Colorado.
- Hess C. G. 1958. The ants of Dallas County, Texas, and their nesting sites; with particular reference to soil texture as an ecological factor. Field and Laboratory 26: 3-72.
- Hunt J. H. and Snelling R. R. 1975. A checklist of the ants of Arizona. Journal of the Arizona Academy of Science 10: 20-23
- Ivanov K., L. Hightower, S. T. Dash, and J. B. Keiper. 2019. 150 years in the making: first comprehensive list of the ants (Hymenoptera: Formicidae) of Virginia, USA. Zootaxa 4554 (2): 532–560.
- Johnson R. Personnal Database. Accessed on February 5th 2014 at http://www.asu.edu/clas/sirgtools/resources.htm
- Johnson, R.A. and P.S. Ward. 2002. Biogeography and endemism of ants (Hymenoptera: Formicidae) in Baja California, Mexico: a first overview. Journal of Biogeography 29:10091026/
- Kansas State Entomology Collection. Dowloaded the 30th of May 2011 at http://biodis.k-state.edu/collections/entomology/
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Kronauer, J.C., R.A. Johnson, J.J. Boomsma. 2007. The Evolution of Multiple Mating in Army Ants. Evolution 61(2):413-422
- MacGown J.A., Hill J.G. and Skvarla M. 2011. New Records of Ants (Hymenoptera: Formicidae) for Arkansas with a Synopsis of Previous Records. Midsouth Entomologist. 4: 29-38
- MacGown, J. 2011. Ants of Tennessee (species list). Accessed 21 April 2011
- MacGown, J.A and J.A. Forster. 2005. A preliminary list of the ants (Hymenoptera: Formicidae) of Alabama, U.S.A. Entomological News 116(2):61-74
- MacGown, J.A. and JV.G. Hill. Ants of the Great Smoky Mountains National Park (Tennessee and North Carolina).
- Mackay W. P., and E. E. Mackay. 2002. The ants of New Mexico (Hymenoptera: Formicidae). Lewiston, New York: Edwin Mellen Press, 400 pp.
- Mackay, W., D. Lowrie, A. Fisher, E. Mackay, F. Barnes and D. Lowrie. 1988. The ants of Los Alamos County, New Mexico (Hymenoptera: Formicidae). pages 79-131 in J.C. Trager, editor, Advances in Myrmecololgy.
- Munsee J. R., W. B. Jansma, and J. R. Schrock. 1986. Revision of the checklist of Indiana ants with the addition of five new species (Hymenoptera: Formicidae). Indiana Academy of Science 95: 265-274.
- O'Keefe S. T., J. L. Cook, T. Dudek, D. F. Wunneburger, M. D. Guzman, R. N. Coulson, and S. B. Vinson. 2000. The Distribution of Texas Ants. The Southwestern Entomologist 22: 1-92.
- Pergande, T. 1895. Mexican Formicidae. Proceedings of the California Academy of Sciences Ser. 2 :850-896
- Quiroz-Robledo, L.N. and J. Valenzuela-Gonzalez. 2006. Las hormigas Ecitoninae (Hymenoptera: Formicidae) de Morelos, México. Revista Biologia Tropical 54(2):531-552
- Rocio, C. B., F. Solis Marin, A. Ortega Rubio, H. Romero Schmidt, and C. Arguelles Mendez, 1993. Absence of response of ant numbers to livestock exclosure at baja-california-sur, Mexico. Arquivos de Biologia e Tecnologia 36: 829-837.
- Roeder K. A., and D. V. Roeder. 2016. A checklist and assemblage comparison of ants (Hymenoptera: Formicidae) from the Wichita Mountains Wildlife Refuge in Oklahoma. Check List 12(4): 1935.
- Smith M. R. 1924. An annotated list of the ants of Mississippi (Hym.) (continued from page 54). Entomological News 35: 77-85.
- Smith M. R. 1927. A contribution to the biology and distribution of one of the legionary ants, Ection schmitti Emery. Annals of the Entomological Society of America 20: 401-404.
- Smith M. R. 1936. A list of the ants of Texas. Journal of the New York Entomological Society 44: 155-170.
- Smith M. R. 1942. The legionary ants of the United States belonging to Eciton subgenus Neivamyrmex Borgmeier. American Midland Naturalist 27: 537-590.
- Snelling G. C. and R. R. Snelling. 2007. New synonymy, new species, new keys to Neivamyrmex army ants of the United States. Memoirs of the American Entomological Institute 80: 459-550
- Van Pelt A. F. 1948. A Preliminary Key to the Worker Ants of Alachua County, Florida. The Florida Entomologist 30(4): 57-67
- Van Pelt A. F. 1958. The ecology of the ants of the Welaka Reserve, Florida (Hymenoptera: Formicidae). Part II. Annotated list. American Midland Naturalist 59: 1-57
- Van Pelt A., and J. B. Gentry. 1985. The ants (Hymenoptera: Formicidae) of the Savannah River Plant, South Carolina. Dept. Energy, Savannah River Ecology Lab., Aiken, SC., Report SRO-NERP-14, 56 p.
- Van Pelt, A. 1983. Ants of the Chisos Mountains, Texas (Hymenoptera: Formicidae) . Southwestern Naturalist 28:137-142.
- Vasquez Bolanos M. 1998. Hormigas (Hymenoptera: Formicidae) colectadas en necrotrampas, en tres localidades de Jalisco, Mexico. Thesis in Biological Science, Las Aguijas, Zapopan, Jalisco, 61 pages.
- Vásquez-Bolaños M. 2011. Lista de especies de hormigas (Hymenoptera: Formicidae) para México. Dugesiana 18: 95-133
- Ward P. S. 1999. Deceptive similarity in army ants of the genus Neivamyrmex (Hymenoptera: Formicidae): taxonomy, distribution and biology of N. californicus (Mayr) and N. nigrescens (Cresson). Journal of Hymenoptera Research 8: 74-97.
- Warren, L.O. and E.P. Rouse. 1969. The Ants of Arkansas. Bulletin of the Agricultural Experiment Station 742:1-67
- Watkins II, J.F. 1982.The army ants of Mexico (Hymenoptera: Formicidae: Ecitoninae). Journal of the Kansas Entomological Society 55(2): 197-247.
- Watkins J. F., II 1972. The taxonomy of Neivamyrmex texanus, n. sp., N. nigrescens and N. californicus (Formicidae: Dorylinae), with distribution map and keys to the species of Neivamyrmex of the United States. Journal of the Kansas Entomological Society 45: 347-372.
- Watkins J. F., II 1976. The identification and distribution of New World army ants (Dorylinae: Formicidae). Waco, Texas: Baylor University Press, 102 pp
- Watkins J. F., II 1985. The identification and distribution of the army ants of the United States of America (Hymenoptera, Formicidae, Ecitoninae). Journal of the Kansas Entomological Society 58: 479-502.
- Wheeler G. C., and J. Wheeler J. 1989. A checklist of the ants of Oklahoma. Prairie Naturalist 21: 203-210.
- Wheeler W. M. 1904. The ants of North Carolina. Bulletin of the American Museum of Natural History. 20: 299-306.
- Wheeler W. M. 1908. The ants of Texas, New Mexico and Arizona. (Part I.). Bulletin of the American Museum of Natural History 24: 399-485.
- Wheeler, G.C. and J. Wheeler. 1985. A checklist of Texas ants. Prairie Naturalist 17:49-64.
- Whitford W. G. 1978. Structure and seasonal activity of Chihuahua desert ant communities. Insectes Sociaux 25(1): 79-88.
- Young J., and D. E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publication. Oklahoma Agricultural Experimental Station 71: 1-42.
- Young, J. and D.E. Howell. 1964. Ants of Oklahoma. Miscellaneous Publications of Oklahoma State University MP-71
- Photo Gallery
- North temperate
- North subtropical
- Tropical
- Ant Associate
- Host of Trachymyrmex arizonensis
- Phorid fly Associate
- Host of Dacnophora pectinatus
- Host of Cremersia sp.
- Host of Ecitomyia wheeleri
- Diapriid wasp Associate
- Host of Ecitovagus gibbus
- Host of Xanionotum hystrix
- Host of Xanionotum wasmanni
- FlightMonth
- Species
- Extant species
- Formicidae
- Dorylinae
- Neivamyrmex
- Neivamyrmex nigrescens
- Dorylinae species
- Neivamyrmex species
- Ssr



