Cyphomyrmex muelleri
| Cyphomyrmex muelleri | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Cyphomyrmex |
| Species: | C. muelleri |
| Binomial name | |
| Cyphomyrmex muelleri Schultz & Solomon, 2002 | |
Cyphomyrmex muelleri live in nests consisting of a single garden chamber. They are typically constructed in steep embankments under the shelter of overhangs or set back underneath the shelter of a rock or root.
Identification
See the description section below and the identification section for the sibling species Cyphomyrmex longiscapus.
Distribution
Schultz et al. (2002) - C. muelleri is currently known almost entirely from the wetter forests of central Panama, but a single specimen collected in wet forest in Ecuador indicates that this species (or a cryptic, closely related species) also occurs in South America.
Latitudinal Distribution Pattern
Latitudinal Range: 12.9599821° to 9.129°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Colombia, Ecuador, Panama (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
|
Schultz et al. (2002) - What little knowledge we have of the ecology of Cyphomyrmex longiscapus in Colombia is consistent with the ecology of the far better studied Panamanian Cyphomyrmex longiscapus and Cyphomyrmex muelleri (Mueller and Weislo 1998; UGM, unpublished). Although no ecological data are reported in Weber’s original (1940) description of C. longiscapus, a subset of the paratype labels indicates that the colony was taken at 1020 m elevation (another label indicates 3200 ft); one paratype label specifies “nesting in rain forest”; and Weber’s notes indicate a collection location of 6°40'N, 75°10'W. Weber (1972) supplies a verbal description and a photograph of the Colombian C. longiscapus type series nest, and provides the additional information that the nest was collected in “a deep, moist ravine in the Andes of Colombia” (p. 57), consistent with Mueller and Weislo (1998), who surveyed 203 nests of C. longiscapus s.l. in Panama and documented a nesting preference for steep embankments along permanent streams. Label data accompanying Brown’s 1971 Colombian collection of C. longsicapus indicate that the nest was encountered at 2000 m elevation in rain forest. The only known Costa Rican specimen, a lone worker, was found inside a dead stick on the ground of an old alluvial terrace close to a river (J. Longino, pers. comm.). Colombian C. longiscapus specimens have been identified from the stomach contents of two poison-dart frogs, Phyllobates aurotaenia and Dendrobates histrionicus (Snelling and Longino, 1992). The only known non-Panamanian C. muelleri specimen was taken in wet forest at sardine bait in Ecuador (C.R.F. Brandão, pers. comm.).
Both species are monogynous and perennial (Mueller and Weislo, 1998; Villesen et al., unpubl.), queens are singly mated (Villesen et al., 1999; Adams et al., unpubl.; Villesen, unpubl.), and both cultivate mycelium gardens (Mueller and Weislo, 1998; Mueller et al., 1998). Nests consist of a single garden chamber and are usually constructed in steep embankments under the shelter of overhangs or set back underneath the shelter of a rock or root. Nest entrance architecture is complex and characteristic (Fig. 8). In Panama, C. longiscapus constructs nests of two distinct, but intergraded, morphologies: (1) the hanging, baglike “swallow’s nest” type (Fig. 8a), sometimes suspended from rock faces; and (2) the “auricle” type (Fig. 8b). Both of these nest types have pronounced, vertically elongate auricle-shaped entrances, but, whereas the “swallow’s nest” type is suspended and the garden chamber is surrounded at the sides by thin (approximately 2 to 5 mm thick) walls constructed by the ants from clay, the simpler “auricle” type nest is set into the soil, the lateral walls of the excavated garden chamber are natural, and only the front walls surrounding the auricle are constructed by the ants. The single described non-Panamanian C. longiscapus nest, from Colombia, was of the swallow’s-nest type (Weber, 1972; Mueller and Weislo, 1998). C. muelleri constructs nests of the auricle type only, but significant differences separate the auricle-type nests of the two species: Only C. longiscapus constructs large, flaring nest auricles that are generally longer in the vertical than in the horizontal dimension (Fig. 8 b; see also Fig. 1 in Mueller and Weislo, 1998). C. muelleri, in contrast, constructs “mouthlike” auricles that possess swollen or thickened rather than flared rims and that are generally longer in the horizontal rather than vertical dimension (Fig. 8 c). Neighboring nests of the two species almost invariably maintain these species-specific features, indicating that differences in auricle architecture are not microhabitat-dependent and confirming that the two species have diverged with respect to nest construction behavior.
Mueller and Wislo (1998) report an average colony size of 29.4 workers for a mixed sample of C. longiscapus and C. muelleri. However, when the colony sizes of these nests are recalculated by species, C. muelleri nests are found to contain twice as many workers (average 43.8 ± 27.57 s.d. workers; range 6-109; N=106) than C. longiscapus nests (average 22.7 ± 12.33 s.d. workers; range 4–58; N=67). This difference in observed nest size may be due in part to sampling bias: Because C. muelleri nests possess less conspicuous entrance auricles than do C. longiscapus nests (Figs. 8b and 8c), smaller colonies of C. muelleri may be less frequently noticed and collected in the field relative to smaller colonies of C. longiscapus. However, in a more recent survey of three populations in central Panama where both species occur sympatrically, conducted in June 1998, a special effort was made to locate and collect smaller nests. In this case the average number of workers per nest was 14.6 ± 9.73 s.d. (range 0–40; N=76) for C. longiscapus and 29.4 ± 24.24 s.d. (range 4–117; N=42) for C. muelleri. Thus, when the problem of size-biased sampling error is addressed and when nests of all sizes are sampled, sympatrically occurring C. muelleri nests are found to contain about twice as many workers as C. longiscapus nests.
For the subset of colonies with alates reported in Mueller and Weislo (1998), C. muelleri averaged 12.4 alates per nest (N=30), whereas C. longiscapus averaged only 7.8 alates per nest (N=56), a pattern paralleling the average worker colony-size differences between the two species. At locations where both species occur in mixed aggregations, alates were found in nests of both species during the early dry season (December to February) of 1996, and also during the wet season in July and August of 1997, suggesting temporal overlap of alate production between the two species at these times. However, collections of both species taken at the same sites in June 1998, at the start of the wet season, yielded only a single alate (a male) from 29 nests of C. muelleri and 101 alates of both sexes from 72 nests of C. longiscapus (Villesen et al., unpubl.). In addition, late dry-season collections from April 2001 yielded only six males from 34 nests of C. muelleri and 111 alates of both sexes from 32 nests of C. longiscapus (UGM, unpubl.). Although these data are inadequate for drawing firm conclusions, they suggest a scenario of partial reproductive isolation in which both C. longiscapus and C. muelleri produce alates during the wet season and early dry season (July to February), but in which only C. longiscapus (and not C. muelleri) produces sexuals during the late dry season and early rainy season (March to June). Additional data are needed from more extensive nest surveys conducted throughout the year, as well as from observations of mating flight times in both species. Pigmentation differences between males of the two species, noted above, may indicate time-of-day separation in alate flight times, a phenomenon known to occur between sympatric, closely related species of Atta (Mariconi, 1970; Weber, 1972; TRS and UGM, pers. obs.). Specifically, the lighter pigmentation in males of C. muelleri suggests nocturnal mating flights, whereas the darker pigmentation in males of C. longiscapus suggests diurnal mating flights.
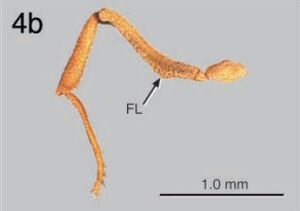
C. longiscapus and C. muelleri are obviously very closely related, and are remarkably similar in terms of ecology, behavior, and morphology. The primary morphological differences separating these species suggest a common evolutionary pattern: Relative to C. longiscapus, C. muelleri appears to be more specialized for cryptic defense. Specifically, the surfaces of the head and alitrunk of C. muelleri are smoother and more rounded than are those of C. longiscapus. Carinae and tubercles are more reduced and the dorsal profile is less interrupted by sutures and grooves (Fig. 2b). This “streamlined” morphology in C. muelleri plausibly reduces the available points of purchase for the mandibles or grasping organs of an attacking, similarly-sized predator (e. g., another arthropod). In contrast to this general trend toward reduced sculpture, but in agreement with the general trend toward more efficient cryptic defense, sculpturing in two features in C. muelleri is increased over that found in C. longiscapus: the posterior postpetiolar tubercles are produced into teeth (Fig. 3b) and the hind femur is equipped with a pair of ventral carinae, forming a ventral groove, and with a ventral lobe (Fig. 4 b). These features, which occur independently in other Cyphomyrmex species (Kempf, 1966), serve to protect vulnerable body parts that are commonly attacked by arthropod predators, particularly other ants; specifically, the postpetiolar tubercles protect the point of articulation between the postpetiole and the gaster; the metafemoral groove receives the tarsus and tibia when the leg is folded in the cryptic-defensive posture; and the metafemoral lobe protects the point of articulation between metatibia and metatarsus.
Morphological features of Cyphomyrmex spp. In general and of C. muelleri in particular suggest adaptations to predation pressure from arthropod-sized predators. Such predation pressure, at least from above-ground (rather than subterranean) hunters, is also suggested by the unusual “auricle” nest-entrance morphology of C. longiscapus and C. muelleri, which may serve as a partial physical or even chemically protected barrier to surface-raiding arthropods, particular predatory ants. Likely examples of such predators include army ants in the subfamily Ecitoninae, which are known to significantly impact Neotropical ant colonies in general (Schneirla, 1971; Rettenmeyer, 1983; Gotwald, 1995; Kaspari, 1996). Published records of army ant predation specifically on fungus-growing ants other than Atta spp. are rare and include no raids on Cyphomyrmex spp. (Cole, 1939; Weber, 1945; Schneirla, 1958, 1971; Fowler, 1977; Mirenda et al., 1980; summarized in LaPolla et al., 2002). That the auricle nest entrance could serve to deter the entry of surface-raiding army (and other) ants is suggested by a single observation in Panama in 1996 in which a Neivamyrmex sp. raiding column, consisting of many thousands of workers, swarmed past the entrances of two C. longiscapus nests. Although many dozens of army ant workers climbed up the outside (i.e., ground-facing) surfaces of the auricles, none ventured onto the auricle rim or onto the frontal (outward) face adjacent to the nest opening (UGM, pers. obs.). If nest-entrance auricle morphology of C. longiscapus (Figs. 8a and 8b) is more efficient at repelling army ants than the auricle morphology of C. muelleri (Fig. 8 c), this could account for stronger selection for the seemingly more effective anti-predatory body morphology in the latter species.
Another known predator of both C. longiscapus and possibly of C. muelleri is the semi-nomadic, socially parasitic, agropredatory ant species Megalomyrmex sp. nov. (Formicidae: Solenopsidini) (Adams et al., 2000b). Based on field and laboratory data, Megalomyrmex sp. nov. colonies aggressively raid C. longiscapus and C. muelleri nests, biting and stinging host-species workers. The raiders eject the resident Cyphomyrmex colony and then occupy and consume the fungus garden over a period of weeks or months, depending on garden size. Unfortunately, this species is known from only a few collections (5 colonies of Megalomyrmex sp. nov. from 344 C. longiscapus/C. muelleri nests collected during 1999 and 2001) (Adams et al., 2000b), and so, without more research, it is impossible to accurately assess the (possibly differential?) predation pressure exerted by this species on C. longiscapus and C. muelleri.
Perhaps the most remarkable difference between C. longiscapus and C. muelleri is that, even though these two species are quite similar biologically and even though they occur sympatrically in the same microhabitats, they consistently employ two very different, distantly related fungal cultivar species. Each of these fungal cultivars is also employed by other, distantly related attine ants that occupy different microhabitats and that are otherwise quite dissimilar biologically (Mueller et al., 1998; Green et al. 2002). Specifically, C. longiscapus shares a narrow group of cultivars of the “Clade 1” type (Mueller et al., 1998) with the sympatric fungus-growing ant Apterostigma auriculatum; molecular data indicate that in one case a cultivar clone has been transferred recently between nests of these two ant species (Mueller et al., 1998). Similarily, C. muelleri shares a narrow group of cultivars of the “Clade 2” type with the sympatric Cyphomyrmex costatus, and multiple cultivar exchanges have occurred between these two ant species (Green et al. 2002). Both A. auriculatum and C. costatus are commonly encountered under logs and rocks on the rain forest floor (UGM, pers. obs.), a very different microhabitat from the embankments preferred by C. longiscapus and C. muelleri. Based on these microhabitat differences, we might expect cultivar
exchanges between C. longiscapus and A. auriculatum, or between C. muelleri and C. costatus, to occur at very low frequencies relative to exchanges between C. longiscapus and C. muelleri, which often occur in mixed aggregations. Yet such across-microhabitat exchanges between the more distantly related attine species are well documented (Mueller et al., 1998; Green et al. 2002). In contrast, in over 400 nest collections in which cultivar species could be identified, C. longiscapus was invariably associated with its own Clade 1 type cultivar, and C. muelleri was invariably associated with its own Clade 2 type cultivar. Thus, cultivar exchanges apparently do not occur between nests of the closely related and physically more proximate C. longiscapus and C. muelleri.
Nesting Habits
Schultz et al. (2002) - (diagnosis): Nest entrance architecture constitutes a generally useful field character for distinguishing between nests of C. muelleri and Cyphomyrmex longiscapus. All nest architecture observations for C. muelleri originate from Panama and, as far as is known, C. muelleri constructs nest entrances of the auricle type only (Fig. 8 c), and not of the swallow’s-nest type (Fig. 8a). Auricle dimensions are summarized in Table 3. In C. muelleri, nest entrance auricles are “mouthlike,” usually wider than high (i. e., longer in the horizontal than in the vertical dimension), and, rather than flared, the auricle rim is merely swollen or thickened beyond the circumference of the base (Fig. 8 c). In his discussion of C. longiscapus, Kempf (1966, p. 167) mentions two specimens collected by W.L. Brown, Jr., and E.S. McCluskey in January 1960 on Barro Colorado Island, Panama (collection no. B-55), which “although basically resembling, I hesitate in definitely ascribing to the present species” [i.e., to C. longiscapus]. Although these specimens (a worker and a gyne) could not be located and thus could not be examined for the present study, they are assignable to C. muelleri based on: (1) the fact that so far only C. muelleri – and not C. longiscapus – has been collected on Barro Colorado Island (UGM, pers. obs.); and, more importantly, (2) Kempf’s succinct description of the worker: “Mesonotum having only the anterior pair of tubercles developed, the rest being flat … Mesoepinotal impression obsolete … Hind femora ventrally lobate and carinate on basal third … [Postpetiole] with a deeper middorsal impression, stronger posterior paired tubercles, which project beyond the mesially deeply excised posterior border” (Kempf, 1966: p. 167). Kempf’s reported worker head measurements (HL=0.72 mm, HW=0.56 mm) fall slightly outside the observed lower range for C. muelleri, but reported worker Weber’s length (1.01 mm) and all gyne measurements (HL=0.88 mm; HW=0.69 mm; WL=1.22 mm) fall within the observed ranges.
Castes
Worker
| |
| . | |
Queen

| |
| . | |
Male
 
| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- muelleri. Cyphomyrmex muelleri Schultz & Solomon, in Schultz, et al. 2002: 336, figs. 1b-7b (w.q.m.) PANAMA, ECUADOR.
- Type-material: holotype worker, 868 paratype workers, 253 paratype queens, 385 paratype males.
- Type-locality: holotype Panama: Barro Colorado I., 14.ii.1996, nest series UGM960214-05 (U.G. Mueller); paratypes: many localities in Panama, extensively listed in Schultz, et al. 2002: 342-343 (appendix), 1 worker Ecuador: Esmeraldas, 10 km. S Atacames, 7.xi.1987 (C.R.F. Brandão & C.D. Bastidas).
- Type-depositories: USNM (holotype); BMNH, CASC, LACM, MCZC, MZSP, USNM (paratypes).
- Distribution: Ecuador, Panama.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Possessing 11 antennal segments and palpal formula 4, 2. Color ranging from yellow to testaceous to fuscous brown. Head and alitrunk uniformly foveate, each fovea usually surrounded by a circlet of whitish “bloom” that resembles the attine actinomycete symbiont (Currie et al., 1999a), the extent of this bloom highly variable across individuals as described above for Cyphomyrmex longiscapus. Pilosity inconspicuous, fine, thin, silvery, and decumbent.
Keying out to C. longiscapus, to which it is very closely related, in the keys of Weber (1940), Kempf (1966), and Snelling and Longino (1992), and sharing with that species the uniquely elongate antennal scapes and weakly expanded frontal lobes. Distinguishable by the following criteria: Vertexal carinae (i. e., paired carinae on the vertex, running parallel to and on either side of the midline) vestigial or absent (in C. longiscapus, strongly produced) (Fig. 1b, VC). Dorsum of the alitrunk in lateral profile smoother and more continuous than in C. longiscapus. In particular, the metanotal groove (“mesoepinotal impression” of Kempf) obsolete, present only as a shallow transverse line (Fig. 2b, MG), so that, in lateral view, the dorsum of mesonotum and propodeum are continuous and uninterrupted by a deep suture such as is present in C. longiscapus. Third intersegmental groove of the thorax (separating the mesopleuron from the metapleuron) incomplete, present dorsally as the vestigial metanotal groove and laterally just above the coxae, but absent in between (Fig. 2 b, IG); in C. longiscapus the groove is complete (Fig. 2a, IG). Posterior tubercles of the postpetiole produced into strong denticles (Fig. 3b, PT), noticeably protruding posterad such that the postpetiole is strongly posteriorly emarginate in dorsal view (Fig. 3 b); in C. longiscapus, the postpetiole is weakly emarginate (Fig. 3a). Hind femur with a pair of strong ventral carina, forming a groove for the reception of the tibia and produced in the basal one-third into a strong ventro-posterior lobe (Fig. 4b, FL), apparently for the protection of the joint between the tibia and tarsus. The ventral femoral groove thus receives the tibia distally and the tarsus basally. Carinae and lobe absent in C. longiscapus (Fig. 4a, arrow).
The following characters are generally useful for distinguishing C. muelleri from C. longiscapus, but, because a minority of specimens in both species possess intermediate states, these additional characters are somewhat less reliable for the identification of C. muelleri: Whitish integumental “bloom” (actinomycete symbiont?), when present elsewhere on the body, also occurring within the antennal scrobe (usually absent in C. longiscapus). Frontal triangle usually laterally compressed, forming a narrow acute triangle or a linear impression (broad and finger-shaped in C. longiscapus). Posterior mesonotal tubercles absent or present as weak carinae (Fig. 2 b, PT); usually well developed in C. longiscapus. Propodeal angle usually absent; the dorsal and declivous faces merged into a continuous curve; propodeal angle usually present in C. longiscapus.
Queen
As in the worker, possessing 11 antennal segments, palpal formula 4, 2, and the C. longiscapus s.l. worker/gyne characters of the elongate antennal scapes and weakly expanded frontal lobes. Color, sculpture, integumental bloom, and pilosity as in the workers.
Generally differing from C. longiscapus in the same character states as the workers except, obviously, for those of the alitrunk. Vertexal carinae vestigial or absent (Fig. 5 b, VC). Postpetiole in dorsal view strongly emarginate posteriorly (as in worker, Fig. 3b). Hind femur with strong ventral carinae and lobe (as in worker, Fig. 4 b, FL).
Male
Antennae with 13 antennal segments, palpal formula 4, 2. Mandibles with four or five teeth, the basal (fifth) tooth sometimes reduced to a rounded basal angle. As in the workers and gynes, head and alitrunk rather uniformly foveate, the foveae occasionally surrounded by circlets of whitish “bloom”; pilosity as in the other castes.
Males of C. muelleri are generally difficult to distinguish from males of C. longiscapus, but they differ in the following characters: As in workers and gynes, the postpetiole of C. muelleri males in dorsal view is strongly emarginate posteriorly (Fig. 7 b); in C. longiscapus males, the postpetiole is very weakly emarginate (Fig. 7a). Propodeal spines longer than those of C. longiscapus males (Fig. 6a, PS), the total spine length exceeding the spine width at the base (Fig. 6 b, PS). All males of C. muelleri examined are uniformly yellow/orange in color, with darker pigmentation restricted to the integument immediately surrounding the ommatidia, as might be expected from a nocturnal or crepuscular flier. Based on three dissections (a male from each of three nests), there is no difference between C. muelleri and C. longiscapus in male genitalic morphology (as described above for C. longiscapus).
Type Material
Holotype (worker): Republic of Panama: Barro Colorado Island; 14 February 1996; U.G. Mueller, collector; Nest series: UGM960214–05. Measurements (in mm): HL=0.85; HW=0.65; WL=1.08; SL=0.79; hind femur length=1.02; greatest diameter of eye=0.17. National Museum of Natural History.
Etymology
It gives us great pleasure to name this species in honor of our friend and colleague Ulrich G. Mueller, who, through diligent field work and exemplary biological study, has pioneered this ideal group of model organisms for attine research.
References
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Cantone S. 2018. Winged Ants, The queen. Dichotomous key to genera of winged female ants in the World. The Wings of Ants: morphological and systematic relationships (self-published).
- Hamilton, N., Jones, T.H., Shik, J.Z., Wall, B., Schultz, T.R., Blair, H.A., Adams, R.M.M. 2018. Context is everything: mapping Cyphomyrmex-derived compounds to the fungus-growing ant phylogeny. Chemoecology 28, 137–144. (doi:10.1007/S00049-018-0265-5).
- Mueller, U.G., Poulin, J., Adams, R.M.M. 2004. Symbiont choice in a fungus-growing and (Attine, Formicidae). Behavioral Ecology 15(2): 357–364 (doi:10.1093/beheco/arh020).
- Schultz T. R., Solomon S. A., Mueller U. G., Villesen P., Boomsma J. J., Adams R. M. M., B. Norden 2002. Cryptic speciation in the fungus-growing ants Cyphomyrmex longiscapus Weber and Cyphomyrmex muelleri Schultz and Solomon, new species (Formicidae, Attini). Insectes Sociaux 49:331-343. 132251.
References based on Global Ant Biodiversity Informatics
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Longino J. T. L., and M. G. Branstetter. 2018. The truncated bell: an enigmatic but pervasive elevational diversity pattern in Middle American ants. Ecography 41: 1-12.
- Longino J. T., and R. K. Colwell. 2011. Density compensation, species composition, and richness of ants on a neotropical elevational gradient. Ecosphere 2(3): 16pp.
- Schultz, T.R., S.A. Solomon, W.G. Mueller, P. Villesen, J.J. Boomsma, R.M.M. Adams and B. Norden. 2002. Cryptic speciation in the fungus-growing ants Cyphomyrmex longiscapus Weber and Cyphomyrmex muelleriSchultz and Solomon, new species (Formicidae,Attini). Insectes Sociaux 49:331-343
- Solomon S. E., C. Rabeling, J. Sosa-Calvo, C. Lopes, A. Rodrigues, H. L. Vasconcelos, M. Bacci, U. G. Mueller, and T. R. Schultz. 2019. The molecular phylogenetics of Trachymyrmex Forel ants and their fungal cultivars provide insights into the origin and coevolutionary history of ‘higher-attine’ ant agriculture. Systematic Entomology 44: 939–956.

