Stictoponera menadensis
| Stictoponera menadensis | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ectatomminae |
| Tribe: | Ectatommini |
| Genus: | Stictoponera |
| Species: | S. menadensis |
| Binomial name | |
| Stictoponera menadensis (Mayr, 1887) | |
| Synonyms | |
| |
Stictoponera menadensis is most commonly found in mesic forested areas. It forages on foliage and nests in a range of locations including in trees and shrubs, rotting wood, tree fern stems, under moss on rocks and in soil. Arboreal nests are constructed in preexisting cavities and sealed with a lining of organic material. Colonies are generally small, with an average of a few hundred workers. Numerous aspects of the biology and natural history of this common species have been studied. Heterick & Kitching (2022) collected this species in a pitfall trap within a lowland dipterocarp forest in Brunei.
| At a Glance | • Gamergate |
Identification
Lattke (2004) - This species is easily confused with Stictoponera bicolor, having similar sculpturing patterns, well developed occipital lobes, and posteriorly placed eyes. S. bicolor differs from S. menadensis by the longitudinally strigulose median area on the promesonotum, the propodeal declivity with a posteromedian raised area and anteriorly diverging sides, and a usually straight metacoxal tooth. S. bicolor generally has more foveolae on the postpetiole, which are deeper and larger in diameter than in S. menadensis; the sides of the fourth abdominal tergite in S. bicolor likewise have larger punctae. Stictoponera bicolor has abundant long standing hairs on the mesosomal dorsum when seen in lateral view with background lighting, in contrast to their virtual absence in S. menadensis.
This species may be part of a species complex (see the nomenclature section below).
Keys including this Species
Distribution
Lattke (2004) - Stictoponera menadensis is a relatively common ant found in the Philippines, southwest into Indonesia, and reaching its western range limit in northeastern peninsular Malaysia. There is a single record from New Guinea. Its range is mostly east of and separated from that of the similar Stictoponera bicolor, though they are sympatric in western Malaysia and Sumatra. S. bicolor is a Southeast Asian ant found from Myanmar eastward, including southern China.
Latitudinal Distribution Pattern
Latitudinal Range: 14.66666667° to -2.183333°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Indo-Australian Region: Borneo, Brunei Darussalam, Indonesia (type locality), Malaysia, New Guinea, Philippines (type locality), Sulawesi.
Oriental Region: India, Thailand.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Habitat
Commonly found in mesic forest.
Biology
Lattke notes:
Most reproduction in S. menadensis is through gamergates (Gobin et al. 1998a).
Stictoponera menadensis from Ulu Gombak nest in the ground and have an average of 500 workers, in contrast to the arboreal habits and smaller nests of S. menadensis from Sulawesi (F. Ito, pers. com.). Thus specimens recognized here as S. menadensis may belong to more than one species, but no consistent morphological differences were detected during the course of this study.
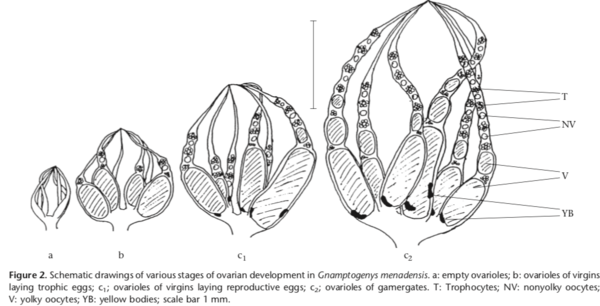
Most habitat labels indicate collection from mesic forested areas. In the Philippines S. menadensis commonly forages on foliage, with nests being found in rotting wood, in tree fern stems, and under moss on rocks (Brown 1954b). In Sulawesi, Indonesia, the ants are arboreal, with foraging and nesting on trees and shrubs. Nests are constructed in preexisting cavities and sealed with a lining of organic material. Colonies are generally small, with an average of 100 workers (Gobin, Peeters, and Billen 1998a). Occasional nesting sites included cavities in limestone rocks and rattan palm leaf shafts. Nests eventually split by budding/fission. Besides hunting for prey, workers also bite flowering buds and lick the exuding sap (Gobin, Peeters, and Billen 1998a). Foragers use chemical trails to home to their nests as well as to recruit to foraging areas (Gobin et al., 1998) by sting-tapping on the substrate. Virgin workers in this species produce trophic eggs (Gobin, Peeters, and Billen 1998b).
Foraging behavior has been studied in this species (also see Poneroid Foraging). The abstract of Johnson et al. (2003) - Stictoponera menadensis is an arboreal nester that forages opportunistically almost exclusively on vegetation, sometimes recruiting others to participate in prey retrieval. The three-dimensional characteristics of vegetation suggest that functions describing recruitment decision thresholds or the pattern of recruitment in arboreal species may differ from those predicted by optimal foraging theory. To examine the effects of prey abundance and distance on the recruitment dynamics of S. menadensis, we baited nests with one termite, five termites or a number of termites between 20 and 40 either near to or far from the entrance and observed the ensuing behaviors. Stictoponera menadensis recruited others when encountering multiple termites regardless of the termite pile’s distance from the nest, although a few individuals remained at the site and defended the resource. The pattern of arrivals at the site indicates that the majority and sometimes all arrivals were recruited from the branch trails. In combination, these results suggest that the architecture of the foraging habitat, which limits available return routes to the nest and thus increases encounter probabilities with potential recruits, shaped the process of information transfer and generated a collective pattern of foraging and prey retrieval.
Workers of Polyrhachis rufipes use the trails of S. menadensis to gain access to sugar sources. When they encounter Stictoponera foragers, P. rufipes workers show a typical aggressive antennal boxing, to which Stictoponera reacts with a submissive behavior (Gobin, Peeters, and Billen 1998b).
Reproduction The majority of colonies of S. menadensis in Sulawesi lack queens and several workers mate and reproduce instead (‘gamergates’). Virgin workers lay morphologically specialized trophic eggs which are fed to larvae (Gobin, Peeters, and Billen 1998c). Some of these virgins switch to male eggs when gamergates are experimentally removed. Three distinct patterns of oogenesis thus result in: (1) trophic eggs; (2) reproductive eggs (unfertilized) laid by virgin workers; and (3) reproductive eggs laid by gamergates, whose ovarioles are always longer than those of virgin workers. Gobin, Peeters, and Billen (1999) investigated the behavioural regulation of ovarian activity in virgin workers by temporarily excluding gamergates. In 12 groups of 35–45 virgins, a few workers became dominant and started to lay reproductive eggs. Once gamergates were reintroduced, sterile workers attacked and immobilized workers with enlarged ovaries (confirmed by dissection of 173 individuals), which often died as a result. Gamergates were never aggressive towards new egg layers. Aggression was not triggered by divergence in colony odours, as it was absent in control experiments in which six colonies were divided in half, with each part containing gamergates, and reunited after 50 days. Our results show that sterile workers discriminate against new egg layers, given that their ovaries are not as developed as those of gamergates. Olfactory detection of different levels of ovarian activity thus appears possible. Mesh experiments indicated that the putative pheromones are nonvolatile and require physical contact for transmission. Aggressive behaviour directed at reproducing workers can be interpreted as worker policing. In S. menadensis, worker policing results in virgins laying only trophic eggs.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
This species is a host for the eucharitid wasp Pogonocharis browni (a parasite) (Universal Chalcidoidea Database) (associate).
Castes
Winged queens exist but they are infrequent in Sulawesi, and most colonies reproduce with several gamergates (Gobin et al. 1998a).
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- menadensis. Ectatomma (Stictoponera) menadensis Mayr, 1887: 539 (footnote) (w.) INDONESIA (Sulawesi).
- Type-material: holotype worker.
- Type-locality: Indonesia: Sulawesi, Menado (Radoszkowski).
- Type-depository: NHMW.
- [Misspelled as manadensis by Jaitrong & Nabhitabhata, 2005: 23.]
- Wheeler, G.C. & Wheeler, J. 1964b: 450 (l.); Imai, et al. 1984: 67 (k.); Lattke, 2004: 131 (q.m.).
- Combination in Gnamptogenys: Brown, 1958g: 228.
- combination in Stictoponera: Emery, 1900d: 663; Camacho, Franco, Branstetter, et al. 2022: 12.
- Status as species: Emery, 1888a: 531; Emery, 1889b: 494 (footnote, in key); Dalla Torre, 1893: 24; Emery, 1900d: 663; Forel, 1901f: 335; Emery, 1901g: 566; Emery, 1911d: 48; Forel, 1911d: 382; Forel, 1911e: 254; Viehmeyer, 1916a: 112; Wheeler, W.M. 1919e: 51; Crawley, 1924: 383; Chapman & Capco, 1951: 30; Brown, 1954h: 2; Brown, 1958g: 228; Baltazar, 1966: 236; Bolton, 1995b: 209; Lattke, 2004: 128 (redescription); Jaitrong & Nabhitabhata, 2005: 23; Pfeiffer, et al. 2011: 35; Bharti, Guénard, et al. 2016: 23; Khachonpisitsak, et al. 2020: 38; Camacho, Franco, Branstetter, et al. 2022: 12.
- Senior synonym of obscura: Brown, 1954h: 2; Bolton, 1995b: 209; Lattke, 2004: 128; Camacho, Franco, Branstetter, et al. 2022: 12.
- Senior synonym of stylata: Brown, 1954h: 2; Bolton, 1995b: 209; Lattke, 2004: 128; Camacho, Franco, Branstetter, et al. 2022: 12.
- Distribution: Indonesia (Kalimantan, Sulawesi, Sumatra); Malaysia (Peninsula, Sabah, Sarawak), Papua New Guinea, Philippines (Leyte, Luzon, Mindanao, Negros), Thailand.
- obscura. Stictoponera menadensis var. obscura Santschi, 1932b: 11 (w.) INDONESIA (Sulawesi).
- Type-material: holotype worker.
- Type-locality: Indonesia: Sulawesi, between Paloe and Koelawi, 4.ii.1929, virgin forest (no collector’s name).
- Type-depository: ISNB.
- Subspecies of menadensis: Chapman & Capco, 1951: 30.
- Junior synonym of menadensis: Brown, 1954h: 2; Bolton, 1995b: 210; Lattke, 2004: 128; Camacho, Franco, Branstetter, et al. 2022: 12.
- stylata. Stictoponera stylata Menozzi, 1925c: 440, fig. 2 (w.) PHILIPPINES (Luzon I.).
- Type-material: holotype worker (probable).
- Type-locality: Philippines: Luzon, Laguna, Mt Makiling (C.F. Baker).
- Type-depository: USNM.
- [Note: status and depository of the holotype are discussed in Lattke, 2004: 133.]
- Status as species: Chapman & Capco, 1951: 31.
- Junior synonym of menadensis: Brown, 1954h: 2; Bolton, 1995b: 211; Lattke, 2004: 128; Camacho, Franco, Branstetter, et al. 2022: 12.
Type Material
- Ectatomma menadensis: Holotype, worker, Menado, Sulawesi, Indonesia, Radoszkowski, Naturhistorisches Museum Wien, Vienna; (see Lattke (2004)).
- Stictoponera stylata: Holotype, worker, Mt. Makiling, Luzon, Philippines, Baker, National Museum of Natural History; (see Lattke (2004)).
- Stictoponera obscura: Holotype, worker, Sulawesi, Indonesia, Royal Belgian Institute of Natural Sciences.
Taxonomic Notes
Lattke (2004) - Occipital lobes prominent, projecting posteroventrally in lateral view; eyes situated on posterior half of head, usually less than one ocular diameter distant from vertex. Mesosomal dorsum mostly densely foveolate to areolate with median longitudinal strip of smooth cuticle, devoid of foveolae on mesonotum; mesosomal dorsal margin mostly devoid of standing hairs in lateral view; propodeal declivity medially with raised posteriorly surface with parallel lateral margins. Metacoxal teeth robust and curved.
There are field data and behavioral information from Fuminori Ito and Bruno Gobin (pers. com.) that suggest the presence of more than one species identifiable as G. menadensis. In Ulu Gombak, Malaysia, they were able to distinguish two forms in the field by the more reddish coloration of one but found the color difference vague in specimens kept in the lab or stored in alcohol. A study of males from this site shows differences in the development of the occipital lobes between the red and nonred forms, but when males from the whole range of G. menadensis are included, these differences collapse. In addition, the number of males available for study is too small to assess the variability of their morphological features.
The type of Stictoponera stylata was present in the collection of J. Chapman but apparently became separated from its label during World War II. Specimens from Chapman's collection made their way to the Museum of Comparative Zoology where Brown (1954b) and Chapman determined that the S. stylata type was among the material they were studying. During the course of the present revision, these specimens were not found in the MCZC, but a series of point mounted workers of S. menadensis from Mt. Makiling, collected by Baker, were found in the National Museum of Natural History. Among this series is a worker with an additional label bearing only the number 20408. This number is similar to those found on "MCZ Cotype" labels for type specimens deposited there during that time (e.g., 20419 for the holotype of Stictoponera taivanensis). On this evidence the aforementioned specimen is considered the probable holotype of S. stylata and has been labeled as such: "Probable holotype."
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Lattke (2004) - Metrics (n = 16): HL 1.30-1.55, HW 1.07-1.23, ML 0.61-0.75 , SL 1.13-1.37, ED 0.25-0.31, WL 1.76-2.11 mm. CI 0.77-0.83, SI 1.03-1.15, MI 0.55-0.61, OI 0.24-0.26. Head with broadly convex lateral margins in frontal view, posterior margin straight with laterally protruding occipital lobes, anterior margin of clypeal lamella forms blunt angle, sometimes projecting anterad as narrow lobe; frons rugulose-foveolate with sharp, roughly longitudinal ridges, foveolae with smooth, convex bottoms; frontal lobe with straight lateral margin; clypeus longitudinally strigose; scape varies from very strigulose to mostly smooth; occipital lobe prominent, projecting posteroventrally in lateral view, lamella convex, low, with ends either angular or convex, usually convex; eye situated posteriorly on head, usually less than one ocular diameter from vertex.
Humeral angle lamellate, pronotal ventral margin narrow, anteroventral corner frequently angular, side densely foveolate with fine strigulae on posterior margin; pronotal dorsum densely foveolate with sharp ridges between depressions, median broad groove present on posterior half; promesonotal suture marked as fine line; mesonotum usually with median longitudinal strip of mostly smooth cuticle; anepisterum narrow, rectangular to cuneiform, usually smooth with some foveolae; katepisternum foveolate, with or without strigulae; metapleuron posteroventrally strigose, anterodorsally with narrow strip of mostly smooth or undulate cuticle; propodeum foveolate, propodeal declivity surrounded posterolaterally by ridges forming denticle or low triangular projection, medially with raised, parallel-sided surface that ends before anterior margin, cuticle surrounding raised area usually smooth. Petiolar node dorsally foveolate; with subquadrate to lobe like ventral process in lateral view; postpetiolar dorsum foveolate anterad, foveolae becoming shallower and sparser posterad, laterally densely foveolate anteriorly; postpetiolar sternum transversely strigulose, laterally foveolate to punctate; dorsum of abdominal segment 4 mostly smooth with scattered punctulae; fourth abdominal sternite strigulose-punctate. Fore coxa transversely strigulose in lateral view; fore tarsus opposite strigil with single prominent basal seta, occasionally followed apically by row of slender setae; metacoxal spine usually curved from the base. Dorsum of thorax and abdominal segments 1-4 with scattered erect to subdecumbent hairs. Head, mesosoma, petiole, and gaster ferruginous brown to brown, gaster frequently darker colored than rest of body.
Queen
Lattke (2004) - Metrics (n = 1): HL 1.27, HW 1.03, ML 0.60, SL 1.14, ED 0.21, WL 1.83 mm. CI 0.81, SI 1.11, MI 0.58, OI 0.20. Pronotum densely foveolate, laterally with narrow mostly smooth band along posterior margin, rest of lateral mesosoma densely foveolate; mesoscutum with shallow foveolae, median longitudinal and shallow sulcus present; propodeal dorsum densely foveolate.
Male
Lattke (2004) - Metrics (n = 1): HL 1.09, HW 1.00, ML 0.53, SL 0.25, ED 0.43, WL 2.00 mm. CI 1.09, SI 0.25, MI 0.53, OI 0.43. Frons mostly foveolate, mandibular dorsum mostly smooth with scattered punctae, clypeus mostly smooth with longitudinal undulations and scattered foveolae laterally. Mesonotum mostly rugulose-punctate in lateral view; anepisternum approximately equal in size to katepisternum; mesoscutum foveolate with large intervening smooth areas, propodeum densely foveolate. Petiolar node densely foveolate. Fore coxa mostly smooth in lateral view, slightly colliculate dorsad, with low transverse strigulae apically.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- n = 21, 2n = 42 (Malaysia) (Goni et al., 1982; Imai et al., 1983; Mariano et al., 2015).
References
- Adams, R.M.M., Wells, R.L., Yanoviak, S.P., Frost, C.J., Fox, E.G.P. 2020. Interspecific Eavesdropping on Ant Chemical Communication. Frontiers in Ecology and Evolution 8. (doi:10.3389/fevo.2020.00024).
- Baer, B. 2011. The copulation biology of ants (Hymenoptera: Formicidae). Myrmecological News 14: 55-68.
- Brown, W. L., Jr. 1954h. A review of the coxalis group of the ant genus Stictoponera Mayr. Breviora. 34:1-10. (page 2, Senior synonym of obscura and stylata)
- Brown, W. L., Jr. 1958g. Contributions toward a reclassification of the Formicidae. II. Tribe Ectatommini (Hymenoptera). Bulletin of the Museum of Comparative Zoology 118: 173-362 (page 228, Combination in Gnamptogenys)
- Camacho, G.P., Franco, W., Branstetter, M.G., Pie, M.R., Longino, J.T., Schultz, T.R., Feitosa, R.M. 2022. UCE phylogenomics resolves major relationships among Ectaheteromorph ants (Hymenoptera: Formicidae: Ectatomminae, Heteroponerinae): A new classification for the subfamilies and the description of a new genus. Insect Systematics and Diversity 6(1): 5; 1–20 (doi:10.1093/isd/ixab026).
- Dhadwal, T., Bharti, H. 2023. A new species of ant genus Stictoponera Mayr, 1887 (Hymenoptera: Formicidae) from India. Asian Myrmecology 16, e016002 (doi:10.20362/am.016002).
- Emery, C. 1900d. Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei. [part]. Ann. Mus. Civ. Stor. Nat. 40[=(2(20): 661-688. (page 663, Combination in Stictoponera)
- Gobin, B., J. Billen & Peeters C. 1999. Policing behaviour towards virgin egg layers in a polygynous ponerine ant. Anim. Behav. 58: 1117-1122 (doi:10.1006/anbe.1999.1245).
- Gobin, B., J. Billen & Peeters C. 2001. Dominance interactions regulate worker mating in the polygynous ponerine ant Gnamptogenys menadensis. Ethology 107: 495–508 (doi:10.1046/j.1439-0310.2001.00689.x).
- Gobin, B., Peeters, C. & Billen, J. 1998a. Colony reproduction and arboreal life in the ponerine ant Gnamptogenys menadensis. Netherlands Journal of Entomology, 48:53-63.
- Gobin, B., Peeters, C., Billen, J. & Morgan, E.D. 1998b. Interspecific trail-following and commensalism between the ponerine ant Gnamptogenys menadensis and the formicine ant Polyrhachis rufipes. J. Insect Behav. 11: 361-369.
- Gobin, B., Peeters, C., Billen, J. 1998. Production of trophic eggs by virgin workers in the ponerine ant Gnamptogenys menadensis. Physiological Entomology 23: 329-336. (doi:10.1046/j.1365-3032.1998.234102.x).
- Heterick, B.E., Kitching, R.L. 2022. The ants (Hymenoptera: Formicidae) of a one-hectare plot of lowland dipterocarp forest. Entomologist’s Monthly Magazine 158(4), 261–272 (doi:10.31184/m00138908.1584.4153).
- Imai, H. T.; Brown, W. L., Jr.; Kubota, M.; Yong, H.-S.; Tho, Y. P. 1984. Chromosome observations on tropical ants from western Malaysia. II. Annu. Rep. Natl. Inst. Genet. Jpn. 34: 66-69 (page 67, karyotype described)
- Ito, F., Hashim, R., Gobin, R. 2021. Colony composition in the Oriental ectatommine ant, Gnamptogenys menadensis in Peninsular Malaysia. Asian Myrmecology 13, e013006 (doi:10.20362/am.013006).
- Johnson, C. A., E. Lommelen, D. Allard, and B. Gobin. 2003. The emergence of collective foraging in the arboreal Gnamptogenys menadensis (Hymenoptera: Formicidae). Naturwissenschaften. 90:332-336.
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Lattke, J. E. 2004. A Taxonomic Revision and Phylogenetic Analysis of the Ant Genus Gnamptogenys Roger in Southeast Asia and Australasia (Hymenoptera: Formicidae: Ponerinae). University of California Publications in Entomology 122: 1-266 (page 128, figs. 29, 45b worker, queen, male described)
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Mayr, G. 1887. Südamerikanische Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 37: 511-632 (page 539, (footnote) worker described)
- Stukalyuk, S., Radchenko, Y., Gonchar, O., Akhmedov, A., Stelia, V., Reshetov, A., Shymanskyi, A. 2021. Mixed colonies of Lasius umbratus and Lasius fuliginosus (Hymenoptera, Formicidae): when superparasitism may potentially develop into coexistence: a case study in Ukraine and Moldova. Halteres 12, 25–48 (doi:10.5281/zenodo.5753121).
- Teixeira, G.A., Barros, L.A.C., Lopes, D.M., de Aguiar, H.J.A.C. 2019. Cytogenetic variability in four species of Gnamptogenys Roger, 1863 (Formicidae: Ectatomminae) showing chromosomal polymorphisms, species complex, and cryptic species. Protoplasma 257, 549–560 (doi:10.1007/s00709-019-01451-6).
- Wheeler, G. C.; Wheeler, J. 1964b. The ant larvae of the subfamily Ponerinae: supplement. Ann. Entomol. Soc. Am. 57: 443-462 (page 450, larva described)
- Wong, T.L., Guénard, B. 2020. Review of ants from the genus Polyrhachis Smith (Hymenoptera: Formicidae: Formicinae) in Hong Kong and Macau, with notes on their natural history. Asian Myrmecology 13: e013001 (doi:10.20362/am.013001).
- Yamane, S., Tanaka, H.O., Hasimoto, Y., Ohashi, M., Meleng, P., Itioka, T. 2021. A list of ants from Lambir Hills National Park and its vicinity, with their biological information: Part II. Subfamilies Leptanillinae, Proceratiinae, Amblyoponinae, Ponerinae, Dorylinae, Dolichoderinae, Ectatomminae and Formicinae. Contributions from the Biological Laboratory, Kyoto University 31, 87–157.
References based on Global Ant Biodiversity Informatics
- Brown W. L., Jr. 1954. A review of the coxalis group of the ant genus Stictoponera Mayr. Breviora 34: 1-10.
- Brown W. L., Jr. 1958. Contributions toward a reclassification of the Formicidae. II. Tribe Ectatommini (Hymenoptera). Bulletin of the Museum of Comparative Zoology 118: 173-362.
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Chapman, J.W. and S.R. Capco. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monographs of the Institute of Science and Technology (Manila) 1: 1- 327
- Crawley W.C. 1924. Ants from Sumatra, with biological notes by Edward Jacobson. Annals and Magazine of Natural History (9)13: 380-409
- Davidson D. W., S. C. Cook, R. R. Snelling and T. H. Chua. 2003. Explaining the Abundance of Ants in Lowland Tropical Rainforest Canopies. Science 300: 969-972.
- Eguchi K., and S. Yamane. 2003. Species diversity of ants (Hymenoptera, Formicidae) in a lowland rainforest, northwestern Borneo. New Entomol. 52(1,2): 49-59.
- Emery C. 1888. Catalogo delle formiche esistenti nelle collezioni del Museo Civico di Genova. Parte terza . Formiche raccolte dal sig. Elio Modigliani in Sumatra e nell'isola Nias. Annali del Museo Civico di Storia Naturale 25: 528-534.
- Emery C. 1901. Formiciden von Celebes. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 14:565-580.
- Emery C. 1911. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125.
- Emery, C.. "Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei." Annali del Museo Civico di Storia Naturale Giacomo Doria (Genova) (2) 20, no. 40 (1900): 661-722.
- Fayle T. M., D. P. Edwards, E. C. Turner, A. J. Dumbrell, P. Eggleton, and W. A. Foster. 2012. Public goods, public services and by-product mutualism in an antfern symbiosis. Oikos 121(8): 1279-1286.
- Forel A. 1901. Nouvelles espèces de Ponerinae. (Avec un nouveau sous-genre et une espèce nouvelle d'Eciton). Revue Suisse de Zoologie 9: 325-353.
- Forel A. 1911. Die Ameisen des K. Zoologischen Museums in München. Sitzungsber. Math.-Phys. Kl. K. Bayer. Akad. Wiss. Münch. 11: 249-303.
- Forel A. 1911. Fourmis nouvelles ou intéressantes. Bull. Soc. Vaudoise Sci. Nat. 47: 331-400.
- Gobin B., C. Peeters, and J. Billen. 1998. Colony reproduction and arboreal life in the ponerine ant Gnamptogenys menadensis (Hymenoptera: Formicidae). Netherlands Journal of Zoology 48(1): 53-63.
- Gobin B., J. Billen, and C. Peeters. 1999. Policing behaviour towards virgin egg layers in a polygynous ponerine ant. Animal Behaviour 58: 11171122.
- Gobin B., J. Billen, and C. Peeters. 2001. Dominance interactions regulate worker mating in the polygynous ponerine ant Gnamptogenys menandensis. Ethology 107: 495-508.
- Hashimoto Y., S. Yamane, and T. Itioka. 1997. A preliminary study on dietary habits of ants in a Bornean rain forest. Japanese Journal of Entomology 65(4): 688-695.
- Hashimoto Y., Y. Morimoto, and M. Mohamed. 2003. Species List of Ground and Leaf Litter Ants Collected in Lower Kinabatangan. Pp 13-18. In Lower Kinabatangan Scientific Expedition 2002, 176 pp. ISBN-13: 983-2369-11-8
- Herwina H., R. Satria, Yaherwandi, and Y. Sakamaki. 2018. Subterranean ant species diversity (Hymenoptera: Formicidae) in educational and biological research forest of universitas andalas, Indonesia. Journal of Entomology and Zoology Studies 6(1): 1720-1724.
- Jaitrong W.; Nabhitabhata, J. 2005. A list of known ant species of Thailand. The Thailand Natural History Museum Journal 1(1): 9-54.
- Janda M., G. D. Alpert, M. L. Borowiec, E. P. Economo, P. Klimes, E. Sarnat, and S. O. Shattuck. 2011. Cheklist of ants described and recorded from New Guinea and associated islands. Available on http://www.newguineants.org/. Accessed on 24th Feb. 2011.
- Kishimoto-Yamata K., F. Hyodo, M. Matsuoka, Y. Hashimoto, M. Kon, T. Ochi, S. Yamane, R. Ishii, and T. Itioka. 2012. Effects of remnant primary forests on ant and dung beetle species diversity in a secondary forest in Sarawak, Malaysia. Journal of Insect Conservation DOI 10.1007/s10841-012-9544-6
- Lattke J. E. 2004. A taxonomic revision and phylogenetic analysis of the ant genus Gnamptogenys Roger in Southeast Asia and Australasia (Hymenoptera: Formicidae: Ponerinae). University of California Publications in Entomology 122: 1-266.
- Lattke, J.E. 2004. A taxonomic revision and phylogenetic analysis of the ant Gnamptogenys Roger in Southeast Asia and Australasia (Hymenoptera: Formicidae: Ponerinae). University of California Publications in Entomology 122: 1-266
- Menozzi C. 1925. Nouvelles fourmis des Philippines. Philippine Journal of Science. 28: 439-451.
- Pfeiffer M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E. Y.; Kohout, R. J. 2011. The Formicidae of Borneo (Insecta: Hymenoptera): a preliminary species list. Asian Myrmecology 4:9-58
- Philpott S.M., P. Bichier, R.A. Rice, and R. Greenberg. 2008. Biodiversity conservation, yield, and alternative products in coffee agroecosystems in Sumatra, Indonesia. Biodivers. Conserv. 17: 1805-1820. Data obtained from Stacy Philpott
- Santschi F. 1932. Résultats scientifiques du voyage aux Indes orientales néerlandaises de LL. AA. RR. le Prince et la Princesse Léopold de Belgique. Hymenoptera. Formicidae. Mémoires du Musée Royal d'Histoire Naturelle de Belgique. (2)4: 11-29.
- Tiwari R.N., B.G. Kundu, S. Roychowdhury, S.N. Ghosh. 1999. Insecta: Hymenoptera: Formicidae. Pp. 211-294 in: Director; Zoological Survey of India (ed.) 1999. Fauna of West Bengal. Part 8. Insecta (Trichoptera, Thysanoptera, Neuroptera, Hymenoptera and Anoplura). Calcutta: Zoological Survey of India, iv + 442 pp.
- Wheeler W. M. 1919. The ants of Borneo. Bulletin of the Museum of Comparative Zoology 63:43-147.
- Yamane S.; Nona, A. R. 1994. Ants from Lambir Hills National Park, Sarawak. Pp. 222-226 in: Inoue, T.; Hamid, A. A. (eds.) 1994. Plant reproductive systems and animal seasonal dynamics. Long-term study of dipterocarp forests in Sarawak. Kyoto: Center for Ecological Research, Kyoto University, vii + 255 pp.