Lasius subumbratus
| Lasius subumbratus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Lasiini |
| Genus: | Lasius |
| Section: | flavus clade |
| Species group: | umbratus |
| Species: | L. subumbratus |
| Binomial name | |
| Lasius subumbratus Viereck, 1903 | |
This species nests in rotten logs and stumps, and under stones. It is a temporary social parasitism. Queens found new colonies by infiltrating an established nest of Lasius neoniger or Lasius pallitarsis, killing the queen and using host workers to care for her initial brood.
| At a Glance | • Temporary parasite |
Photo Gallery
Identification
A close relative of Lasius umbratus sympatric with that species over most of its range and best distinguished from it by differences in body pilosity.
Mackay and Mackay (2002) - These ants are yellow or pale brown, with small eyes (about 0.13 - 0.15 mm in greatest diameter, with about 50 ommatidia). The longest hairs on the posterior half of the first gastral tergite are at least 0.60 times as long as the maximum width of the posterior tibia at mid length. The erect hairs are dense enough that their length is less than the distance between their tips. The apex of the petiole is flat or slightly concave, as seen from the front.
Ellison et al. (2012)- Lasius subumbratus is most easily confused with the similarly hairy Lasius minutus. But the hairs on the gaster of L. subumbratus are only 60-80% as long as the hind tibia is wide, whereas the hairs on the gaster of L. minutus are longer than the hind tibia is wide. It is also possible to confuse L. subumbratus with Lasius umbratus, but L. subumbratus has a convex-topped petiole (viewed from the front or back) whereas L. umbratus has a concave-topped petiole.
Keys including this Species
- Key to Lasius Nearctic workers with long maxillary palpi
- Key to Lasius males
- Key to Lasius queens
- Key to North American Lasius Species
- Key to New England Lasius
Distribution
This northern species ranges from the Canadian Maritimes to western North America. In New England, it is confined to cold-temperate and sub-boreal areas and to date has been collected only from Maine (Ellison et al., 2012).
Latitudinal Distribution Pattern
Latitudinal Range: 53.917° to 31.882254°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: Canada, United States (type locality).
Neotropical Region: Mexico.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
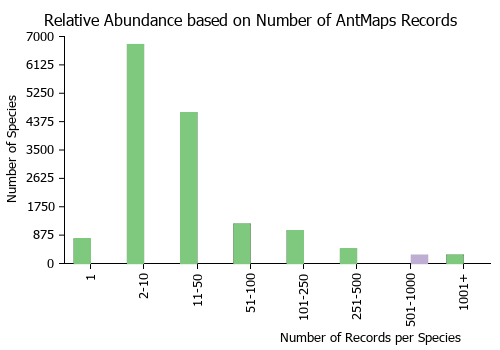
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
|
Biology
Wilson (1955) - Cole's New Mexican collections were made under stones in a variety of habitats, including a dry, open slope, an alpine meadow, and spruce-aspen, spruce-pine, and pine-aspen forests. Wheeler (1917b) found it common at Cloudcroft, New Mexico, under stones in pine forest. I found two colonies in pine-fir forest on a southern slope of the San Francisco Mountains of Arizona, one under a stone and one under a rotting log. In the southern Rocky Mountains Lasius subumbratus is clearly a high-elevation species; thus far it has been found only well above the lower elevational limits of sympatric populations of Lasius pallitarsis, Lasius neoniger, Lasius crypticus, and Lasius sitiens, and it ranges at least to the upper elevational limits of these species. In New Mexico Cole encountered it between 7400 and 10,000 feet, and at Cloudcroft Wheeler was unable to find it below 9000 feet. My Arizona colonies were found at about 8000 feet.
Wheeler's studies at Cloudcroft (1917b) leave no doubt that subumbratus is a temporary social parasite of pallitarsis. The alternate host, Lasius neoniger, which he found nesting in open, dry situations, may be pallitarsis also. I have found nothing but pallitarsis in his collections from this locality, and this species was the only member of the subgenus encountered during my own brief visit there. According to Wheeler, subumbratus is abundant enough at Cloudcroft to flood the pallitarsis nests with queens at the time of the nuptial flight. At one spot after a nuptial flight (occurring July 6 or 7) he found dealate subumbratus queens in nearly every nest of the host species uncovered. He observed that these queens approach the pallitarsis workers in a conciliatory manner, that they are often rebuffed at first, and that they sometimes hide in the vicinity of brood piles prior to adoption. He saw one queen in the act of stealing a host pupa and another carrying an uninjured host worker. The latter incident is reminiscent of the conditioning behavior of Lasius umbratus more recently described by several European authors (q.v.). Several pallitarsis colonies with recently adopted parasite queens were found, as well as two colonies containing workers of both species. From these observations Wheeler drew the conclusion that the subumbratus queens are by nature conciliatory, but still find it necessary to acquire some amount of the host nest odor in order to secure final adoption.
Additional evidence is available to indicate that pallitarsis is the principal, if not the only, host. Cole has found mixed subumbratus-pallitarsis colonies at Tesuque Canyon and Eagle Nest, New Mexico; the parasite workers from the latter locality are minimas and much smaller than the associated host workers. In another nest at the second locality a single dealate subumbratus queen was found with pallitarsis workers. Finally, there is in the Museum of Comparative Zoology a series of 6 dealate subumbratus queens associated with pallitarsis (Bedford, Nova Scotia; W. Reig leg.; MCZ).
In New Mexico (Mackay and Mackay 2002) - Ranging from meadows and dry open slopes through mixed forest into ponderosa pine - riparian up to spruce forests. This species nests under stones or logs in loam soils with rocks. It is a temporary social parasite of Lasius pallitarsis. Two dealate females were found in one nest, suggesting that this species may be polygynous. Brood was found in nests in March and August, reproductives were found in nests in July and August. Dealate females were collected in July and August. It tends mealybugs.
Flight Period
| X | X | ||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
Source: antkeeping.info.
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
 Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Castes
Worker
   
| |
| Worker. . | Owned by Museum of Comparative Zoology. |
Images from AntWeb
   
| |
| Worker. Specimen code casent0102807. Photographer Jen Fogarty, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Queeen
   
| |
| Queen. . | Owned by Museum of Comparative Zoology. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- subumbratus. Lasius umbratus subsp. subumbratus Viereck, 1903: 73 (q.) U.S.A. Wheeler, W.M. 1910e: 238 (w.m.). Combination in L. (Chthonolasius): Emery, 1925b: 235. Raised to species: Creighton, 1950a: 424. See also: Wheeler, W.M. 1917h: 167; Wilson, 1955a: 175.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Wilson (1955) - (1) Gastric pilosity longer and denser than in Lasius umbratus. The hairs-on the first tergite with a maximum length of about 0.10 mm., or about 0.6 X the maximum width of the hind tibia at its midlength, mostly decumbent-subdecumbent (occasionally tending to suberect), and dense enough for the individual hairs to overlap one another widely. Scapes, femora, and tibiae with dense, predominantly decumbent pubescence and occasional standing hairs (umbratus in all populations with sparser pubescence, often appressed, and the North American population always lacking standing hairs). Gaster with very sparse pubescence, not obscuring in any way the shining cuticular surface.
(2) Eyes smaller than in North American umbratus, the HW-EW regression zones of the two species well separated, although no single absolute measurement will suffice to separate all the series. The Eurasian population of umbratus connects and overlaps the two. (3) Size averaging larger than in umbratus, PW range 0.55-0.85 mm.
(4) Body and appendages uniformly medium yellow, lighter than most North American umbratus and Lasius minutus.
Queen
Wilson (1955) - (1) Pilosity on anterior three gastric tergites very long (maximum length 0.24-0.27 mm., or approximately the maximum width of the hind tibia at midlength), silvery yellow, and predominantly decumbent-subdecumbent. Similar erect hairs form a fringe around the entire dorsal and lateral margins of the petiole; these are often curved toward their tips. Pilosity of alitrunk mostly limited to the dorsal surface, from the posterior margin of the pronotum to the dorsal face of the propodeum, averaging shorter than on the gaster and petiole, maximum length about 0.24 mm., subdecumbent to erect, often curved or sinuate. Pilosity of head mostly limited to the occipital zone, averaging shorter than on the alitrunk, maximum length about 0.18 mm., predominantly subdecumbent-suberect and often curved.
(2) Averaging larger and with proportionately longer appendages than the sympatric North American population of umbratus. Extreme range of HW with attendant SI of series examined 1.56 mm., 85; 1.74 mm., 83.
(3) Color averaging lighter than in umbratus. Body typically medium yellowish brown, head somewhat lighter; appendages light yellowish brown.
Male
(1) Gastric pilosity similar to that of worker in form and inclination, but sparser, more often subdecumbent, and showing only limited overlap between individual hairs; maximum length of hairs about 0.13 mm., or 0.9-1.1 X the maximum width of the hind tibia at midlength. Maximum length of the hairs of the posterior two-thirds of the clypeus 0.12 mm., or slightly less than 0.10 X the HW.
(2) Averaging larger than in umbratus; maximum range in all series studied 1.05-1.17 mm.
(3) Genitalia similar in all respects to those of umbratus.
Type Material
HOLOTYPE. A winged queen in the Academy of Natural Sciences, Philadelphia, in good condition and showing all of the characters used in the diagnosis above. A paratopotype queen, recently compared with the holotype, is in the Museum of Comparative Zoology.
References
- Boudinot, B.E., Borowiec, M.L., Prebus, M.M. 2022. Phylogeny, evolution, and classification of the ant genus Lasius, the tribe Lasiini and the subfamily Formicinae (Hymenoptera: Formicidae). Systematic Entomology 47, 113-151 (doi:10.1111/syen.12522).
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Creighton, W. S. 1950a. The ants of North America. Bulletin of the Museum of Comparative Zoology 104: 1-585 (page 424, raised to species)
- Ellison, A.M., Gotelli, N.J., Farnsworht, E.J., Alpert, G.D. 2012. A Field Guide to the Ants of New England. Yale University Press, 256 pp.
- Emery, C. 1925d. Hymenoptera. Fam. Formicidae. Subfam. Formicinae. Genera Insectorum 183: 1-302 (page 235, Combination in L. (Chthonolasius))
- Mackay, W. P. and E. Mackay. 2002. The ants of New Mexico (Hymenoptera: Formicidae). Edwin Mellen Press, Lewiston, NY.*Viereck, H. L. 1903. Hymenoptera of Beulah, New Mexico. [part]. Trans. Am. Entomol. Soc. 29: 56-87 (page 73, queen described)
- Wheeler, W. M. 1910h. The North American forms of Lasius umbratus Nylander. Psyche (Camb.) 17: 235-243 (page 238, worker, male described)
- Wheeler, W. M. 1917h. The temporary social parasitism of Lasius subumbratus Viereck. Psyche (Camb.) 24: 167-176 (page 167, see also)
- Wilson, E. O. 1955a. A monographic revision of the ant genus Lasius. Bulletin of the Museum of Comparative Zoology 113: 1-201 (page 175, see also)
References based on Global Ant Biodiversity Informatics
- Allred D. M. 1982. Ants of Utah. The Great Basin Naturalist 42: 415-511.
- Allred, D.M. 1982. The ants of Utah. Great Basin Naturalist 42:415-511.
- Bestelmeyer B. T., and J. A. Wiens. 2001. Local and regional-scale responses of ant diversity to a semiarid biome transition. Ecography 24: 381-392.
- Borchert, H.F. and N.L. Anderson. 1973. The Ants of the Bearpaw Mountains of Montana (Hymenoptera: Formicidae). Journal of the Kansas Entomological Society 46(2):200-224
- Carroll T. M. 2011. The ants of Indiana (Hymenoptera: Formicidae). Master's Thesis Purdue university, 385 pages.
- Cole A. C., Jr. 1942. The ants of Utah. American Midland Naturalist 28: 358-388.
- Cover S. P., and R. A. Johnson. 20011. Checklist of Arizona Ants. Downloaded on January 7th at http://www.asu.edu/clas/sirgtools/AZants-2011%20updatev2.pdf
- Del Toro, I. 2010. PERSONAL COMMUNICATION. MUSEUM RECORDS COLLATED BY ISRAEL DEL TORO
- Drummond F. A., A. M. llison, E. Groden, and G. D. Ouellette. 2012. The ants (Formicidae). In Biodiversity of the Schoodic Peninsula: Results of the Insect and Arachnid Bioblitzes at the Schoodic District of Acadia National Park, Maine. Maine Agricultural and forest experiment station, The University of Maine, Technical Bulletin 206. 217 pages
- Gregg, R.T. 1963. The Ants of Colorado.
- Ivanov K., L. Hightower, S. T. Dash, and J. B. Keiper. 2019. 150 years in the making: first comprehensive list of the ants (Hymenoptera: Formicidae) of Virginia, USA. Zootaxa 4554 (2): 532–560.
- Johnson R. Personnal Database. Accessed on February 5th 2014 at http://www.asu.edu/clas/sirgtools/resources.htm
- Kannowski P. B. 1956. The ants of Ramsey County, North Dakota. American Midland Naturalist 56(1): 168-185.
- La Rivers I. 1968. A first listing of the ants of Nevada. Biological Society of Nevada, Occasional Papers 17: 1-12.
- Lidgren, B.S. and A.M. MacIsaac. 2002. A Preliminary Study of Ant Diversity and of Ant Dependence on Dead Wood in Central Interior British Columbia. USDA Forest Service Gen. Tech. Rep. PSW-GTR-181.
- Lindgren, B.S. and A.M. MacIsaac. 2002. Ant dependence on dead wood in Central Interior British Columbia. USDA Forest Service Gen. Tech. Rep.PSW-GTR-181
- Longino, J.T. 2010. Personal Communication. Longino Collection Database
- Mackay W. P., and E. E. Mackay. 2002. The ants of New Mexico (Hymenoptera: Formicidae). Lewiston, New York: Edwin Mellen Press, 400 pp.
- Mackay, W., D. Lowrie, A. Fisher, E. Mackay, F. Barnes and D. Lowrie. 1988. The ants of Los Alamos County, New Mexico (Hymenoptera: Formicidae). pages 79-131 in J.C. Trager, editor, Advances in Myrmecololgy.
- Merle W. W. 1939. An Annotated List of the Ants of Maine (Hymenoptera: Formicidae). Entomological News. 50: 161-165
- Ouellette G. D., F. A. Drummond, B. Choate and E. Groden. 2010. Ant diversity and distribution in Acadia National Park, Maine. Environmental Entomology 39: 1447-1556
- Procter W. 1938. Biological survey of the Mount Desert Region. Part VI. The insect fauna. Philadelphia: Wistar Institute of Anatomy and Biology, 496 pp.
- Rees D. M., and A. W. Grundmann. 1940. A preliminary list of the ants of Utah. Bulletin of the University of Utah, 31(5): 1-12.
- Vasquez-Bolanos M. 2011. Checklist of the ants (Hymenoptera: Formicidae) from Mexico. Dugesiana 18(1): 95-133.
- Vásquez-Bolaños M. 2011. Lista de especies de hormigas (Hymenoptera: Formicidae) para México. Dugesiana 18: 95-133
- Wheeler G. C., J. N. Wheeler, and P. B. Kannowski. 1994. Checklist of the ants of Michigan (Hymenoptera: Formicidae). The Great Lakes Entomologist 26(4): 297-310
- Wheeler G. C., and J. Wheeler. 1986. The ants of Nevada. Los Angeles: Natural History Museum of Los Angeles County, vii + 138 pp.
- Wheeler W. M. 1917. The mountain ants of western North America. Proceedings of the American Academy of Arts and Sciences 52: 457-569.
- Wheeler, G.C. and J. Wheeler. 1988. A checklist of the ants of Montana. Psyche 95:101-114
- Wheeler, G.C. and J. Wheeler. 1988. A checklist of the ants of Wyoming. Insecta Mundi 2(3&4):230-239
- Wheeler, G.C., J. Wheeler and P.B. Kannowski. 1994. CHECKLIST OF THE ANTS OF MICHIGAN (HYMENOPTERA: FORMICIDAE). Great Lakes Entomologist 26:1:297-310
- Wheeler, G.C., J. Wheeler, T.D. Galloway and G.L. Ayre. 1989. A list of the ants of Manitoba. Proceedings of the Entomological Society of Manitoba 45:34-49
- Wilson E. O. 1955. A monographic revision of the ant genus Lasius. Bulletin of the Museum of Comparative Zoology 113: 1-201
- Wing M. W. 1939. An annotated list of the ants of Maine (Hymenoptera: Formicidae). Entomological News 50:161-165.
- Pages using DynamicPageList3 parser function
- Ant Associate
- Host of Lasius neoniger
- Host of Lasius pallitarsis
- Temporary parasite
- Photo Gallery
- North temperate
- North subtropical
- FlightMonth
- Species
- Extant species
- Formicidae
- Formicinae
- Lasiini
- Lasius
- Lasius subumbratus
- Formicinae species
- Lasiini species
- Lasius species
- Need Overview
- Need Body Text


