Hymenoptera Sex Determination
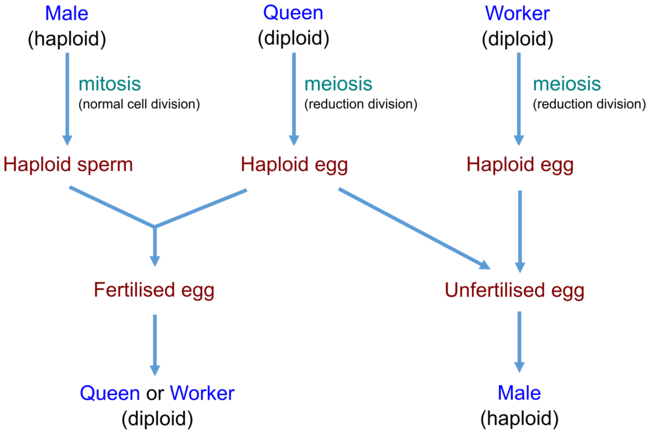
Within ants, females (queens, workers) are produced from fertilised eggs (which contain two sets of chromosomes) and are diploid, while males are produced from unfertilised eggs (which contain a single set of chromosomes) and are haploid. This sex-determination system is termed haplodiploidy (or sometimes arrhenotoky, especially when discussing parthenonenesis). This results in males having half the number of chromosomes that females have.
From a mechanistic perspective (Miyakawa et al., 2018), a single, complementary sex-determination (sl-CSD) locus functions as the primary sex-determination signal. In haplodiploid taxa, females are heterozygous at the CSD locus while males are hemi/homozygous.
Haplodiploidy occurs in all species of Hymenoptera (bees, ants, wasps and sawflies) and Thysanoptera ('thrips'), as well as sporadically in some spider mites, Hemiptera, Coleoptera (bark beetles) and rotifers.
Diploid Males
It should be noted that while in the vast majority of ants males arise from unfertilised eggs (haploid, 1n), in a handful of species diploid males are known. These diploid males generally show low viability, or fail to produce viable offspring as they are unable to mate properly or because they are sterile. Additionally, while some diploid males can produce viable sperm, their sperm is diploid rather than haploid and the resulting offspring are triploid and sterile.
Definitions
- Arrhenotoky or Arrhenotokous Parthenogenesis
- A form of parthenogenesis (reproduction without mating) in which unfertilised eggs develop into males, where parthenogenesis itself produces exclusively females.
- Mitosis
- The process where a single cell divides once to form two identical daughter cells (cell division). The major purpose of mitosis is for growth and to replace existing, aging cells.
- Meiosis
- The process where a single cell divides twice to produce four cells (gametes), each containing half the original genetic material (chromosomes, DNA). These cells are sex cells – sperm in males, eggs in females.
Sex Determination Studies
- Miyakawa, M. O., K. Tsuchida, and H. Miyakawa. 2018. The doublesex gene integrates multi-locus complementary sex determination signals in the Japanese ant, Vollenhovia emeryi. Insect Biochemistry and Molecular Biology. 94:42-49 (doi:10.1016/j.ibmb.2018.01.006).
Abstract A female diploid, male haploid sex determination system (haplodiploidy) is found in hymenopteran taxa, such as ants, wasps, bees and sawflies. In this system, a single, complementary sex-determination (sl-CSD) locus functions as the primary sex-determination signal. In the taxa that has evolved this system, females and males are heterozygous and hemi/homozygous at the CSD locus, respectively. While the sl-CSD system enables females to alter sex ratios in the nest, it carries a high cost in terms of inbreeding, as individuals that are homozygous at the CSD locus become sterile diploid males. To counter this risk, some of hymenopteran species have evolved a multi-locus CSD (ml-CSD) system, which effectively reduces the proportion of sterile males. However, the mechanism by which these multiple primary signals are integrated and how they affect the terminal sex-differentiation signal of the molecular cascade have not yet been clarified. To resolve these questions, we examined the molecular cascade in the Japanese ant Vollenhovia emeryi, which we previously confirmed has two CSD loci. Here, we showed that the sex-determination gene, doublesex (dsx), which is highly conserved among phylogenetically distant taxa, is responsible for integrating two CSD signals in V. emeryi. After identifying and characterizing dsx, genotypes containing two CSD loci and splicing patterns of dsx were found to correspond to the sexual phenotype, suggesting that two primary signals are integrated into dsx. These findings will facilitate future molecular and functional studies of the sex determination cascade in V. emeryi, and shed light on the evolution and diversification of sex
- Miyakawa, M.O., Mikheyev, A.S. 2015. QTL mapping of sex determination loci supports an ancient pathway in ants and honey bees. PLoS Genet 11(11): e1005656 (doi:10.1371/journal. pgen.1005656).
Abstract Sex determination mechanisms play a central role in life-history characteristics, affecting mating systems, sex ratios, inbreeding tolerance, etc. Downstream components of sex determination pathways are highly conserved, but upstream components evolve rapidly. Evolutionary dynamics of sex determination remain poorly understood, particularly because mechanisms appear so diverse. Here we investigate the origins and evolution of complementary sex determination (CSD) in ants and bees. The honey bee has a well-characterized CSD locus, containing tandemly arranged homologs of the transformer gene [complementary sex determiner (csd) and feminizer (fem)]. Such tandem paralogs appear frequently in aculeate hymenopteran genomes. However, only comparative genomic, but not functional, data support a broader role for csd/fem in sex determination, and whether species other than the honey bee use this pathway remains controversial. Here we used a backcross to test whether csd/fem acts as a CSD locus in an ant (Vollenhovia emeryi). After sequencing and assembling the genome, we computed a linkage map, and conducted a quantitative trait locus (QTL) analysis of diploid male production using 68 diploid males and 171 workers. We found two QTLs on separate linkage groups (CsdQTL1 and CsdQTL2) that jointly explained 98.0% of the phenotypic variance. CsdQTL1 included two tandem transformer homologs. These data support the prediction that the same CSD mechanism has indeed been conserved for over 100 million years. CsdQTL2 had no similarity to CsdQTL1 and included a 236-kb region with no obvious CSD gene candidates, making it impossible to conclusively characterize it using our data. The sequence of this locus was conserved in at least one other ant genome that diverged >75 million years ago. By applying QTL analysis to ants for the first time, we support the hypothesis that elements of hymenopteran CSD are ancient, but also show that more remains to be learned about the diversity of CSD mechanisms.
| ||||||||||||||||||||||||||