Strumigenys rogeri
| Strumigenys rogeri | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Strumigenys |
| Species: | S. rogeri |
| Binomial name | |
| Strumigenys rogeri Emery, 1890 | |
| Synonyms | |
| |
Strumigenys rogeri is a very small ant (total length ~ 2.5 mm) that nests in and under dead wood and preys on tiny soil arthropods. This species has spread to many parts of the world through human commerce. However, because S. rogeri workers are so small and slow moving, and they become motionless when disturbed, most people, including field biologists, remain unaware of their presence. Strumigenys rogeri apparently originated in tropical Africa, where its closest relatives all live, but it has become wide-spread on tropical islands of the Indo-Pacific and the West Indies, and in peninsular Florida. Outside of Africa and Florida, there are only a small number of continental records of S. rogeri, including a few from South and Central America and just one from continental Asia, in peninsular Malaysia. It is unclear whether S. rogeri has not yet spread to these continental areas, whether continental ants have competitively excluded S. rogeri, or whether these ants have been simply overlooked in surveys of diverse continental faunae. There is little information on what impact S. rogeri may be having on the native mesofauna in its exotic range. (Wetterer 2012)
| At a Glance | • Secretly invasive • Parthenogenetic |
Identification
Bolton (2000) - A member of the rogeri complex in the Strumigenys rogeri -group. In both Strumigenys bernardi and Strumigenys vazerka the mandibles are slender and obviously bowed outward. On each mandible the proximal preapical tooth is elongate, slender and spiniform, shallowly recurved and usually distinctly longer than the maximum width of the mandible. Distal preapical teeth much smaller and inconspicuous, that on the right mandible usually completely concealed by the left apicodorsal tooth at full closure. Distal preapical tooth of left mandible frequently missing in bernardi. Most samples of bernardi can quickly be discriminated from vazerka (and also rogeri) by this lack, as the distal preapical tooth of the left mandible is always present in vazerka. However, in some populations of bernardi a distal preapical denticle or small tooth is retained. These can be identified as the propodeal dorsum is reticulate-punctate in bernardi, smooth and shining in vazerka.
Bolton (1983) - Among the members of the rogeri-complex in which the preocular notch is strongly developed and extends onto the ventral surface of the head as a transverse groove or impression, rogeri is characterized by its simple dentition (without intercalary teeth in the apical fork and with a full complement of preapical teeth), relatively long straight mandibles, long antennal scapes, presence of pronotal flagellate hairs, and presence of characteristically shaped upper scrobe margins which lack a projecting laminar rim or flange.
Bharti & Akbar (2013), India - The species is easily recognized in the Indian fauna as it is the only one to have the ventrolateral margin of the head deeply indented immediately in front of the eye, so deeply that the anterior portion of the eye is detached from the side of the head. Ventral surface of head with a transverse preocular impression that is posterior to and separate from the postbuccal groove. Preapical dentition of each mandible of two articles; with a preapical tooth and a denticle (Bolton, 2000).
Keys including this Species
- Key to Micronesian Ants
- Key to Strumigenys of India
- Key to Afrotropical Strumigenys
- Key to Neotropical and Nearctic Strumigenys
- Key to Strumigenys of East Asia
- Key to Malagasy Strumigenys
- Key to US Strumigenys species
- Key to Strumigenys of Hispaniola
Distribution
Native: Afrotropical region, known from Ivory Coast to Zanzibar Archipelago (Tanzania) and south to Angola (Tang et al., 2019).
Introduced: A widespread species in multiple biogeographic realms. For a full global account, see antmaps.org (Janicki et al. 2016; Guénard et al. 2017). Here, the Asian distribution is presented for the Oriental realm (Hong Kong; Kerala [India]; Java and Sumatra [Indonesia], Peninsular and East Malaysia [Malaysia], Philippines, and Taiwan). We can also confirm the presence of this species from Vietnam, which was previously reported by Zryanin (2011) (Tang et al., 2019).
In Florida (where it is introduced) it is a common species in bayheads and swamp forest as far north as northern Orange County. Pest status: none. First published Florida record: Deyrup and Trager 1984; earlier specimens: 1965. (Deyrup, Davis & Cover, 2000.)
The record of this tramp species in Hong Kong is not surprising considering its widespread range in nearby countries (Philippines, Taiwan, and Vietnam), which have relatively similar climatic conditions. However, a single alate has been collected from a mangrove habitat, an unlikely habitat for this species, and no workers have been collected in Hong Kong. Nonetheless, the record from Hong Kong is the first observation of this species for mainland China (Tang et al., 2019).
Latitudinal Distribution Pattern
Latitudinal Range: 6.417222222° to -6.26755°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Afrotropical Region: Angola, Burundi, Cameroun, Comoros, Gabon, Ghana, Ivory Coast, Nigeria, United Republic of Tanzania.
Australasian Region: New Caledonia.
Indo-Australian Region: Borneo, Fiji, Indonesia, Malaysia, Marshall Islands, Micronesia (Federated States of), New Guinea, Niue, Northern Mariana Islands, Philippines, Samoa, Singapore, Solomon Islands, Tonga, Vanuatu, Wallis and Futuna Islands.
Malagasy Region: Madagascar, Mauritius, Mayotte, Réunion, Seychelles.
Nearctic Region: Canada, United States.
Neotropical Region: Barbados, British Virgin Islands, Costa Rica, Cuba, Dominican Republic, Greater Antilles, Grenada, Guadeloupe, Haiti, Honduras, Lesser Antilles, Mexico, Netherlands Antilles, Puerto Rico, Saint Lucia.
Oriental Region: India, Thailand, Vietnam.
Palaearctic Region: China, Morocco, United Kingdom of Great Britain and Northern Ireland.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
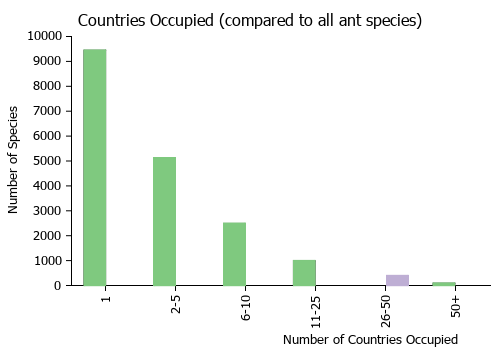
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Strumigenys rogeri is a well known and very efficient tramp species, probably of West African origin but very widely distributed in the tropics by human commerce. It has also been recorded from hothouses and other constantly heated buildings in the temperate zone. Brown (1954) gives observations on the biology of rogeri which were made by Wilson in Cuba. In West Africa the species usually nests in rotten wood on the ground or under the bark of larger fallen trunks or branches, but on occasion it will nest directly in the soil or in wood which has crumbled almost to powder. The Neotropical distribution of rogeri is summarized by Brown (1962b) and Kempf (1972), and the Pacific distribution by Wilson & Taylor (1967).
Brown (1954): "I am fortunate also in having important notes on the biology of S. rogeri made by E. O. Wilson during his stay in Cuba in the summer of 1953.
Wilson took his observation colony at San Vicente, Pinar del Rio, Cuba, from a small nest under a rotten limb lying on well shaded ground. The galleries extended into the wood itself. Transferred to a small plaster observation nest, the workers readily captured numbers of entomobryoid collembolans proffered; campodeids up to four times the length of the ants were also always accepted and, like the entomobryoids, were fed to the larvae. Also observed to be chewed by the larvae after capture were a small psocopteran, a small ichneumonid wasp, and a small, injured embiopteran that had previously been ignored by a colony of Smithistruma nigrescens (= Strumigenys nigrescens) Wheeler. A symphylan and a pseudoscorpion, one each, were accepted and eaten by the larvae, but only after lengthy contact with the ants. Other specimens of these last two groups seem to have been refused by the larvae after capture on some occasions. A small polydesmid millipede was also captured, but soon rejected by larvae and workers. Consistently avoided or ignored when offered in the intimate confines of the observation nest were mites, nasute and other termites, small isopods, poduroid collembolans, adult staphylinid and sylvanid beetles of small size, a small campodeiform beetle larva, and dead mosquitoes, though the beetles mentioned disappeared from the nest and may possibly have been eaten.. Drosophila adults werc caught by the adults, but later discarded.
Entomobryoid collembolans seemed to be the usual and preferred prey fed to the larvae, although campodeids were never refused. In feeding habits, therefore, S. rogeri follows the generic habit of collembolan predation but, like some other widespread dacetine species, it will also accept a variety of other small arthropods, particularly campodeids, when available. In hunting, or when disturbed, the workers and females open the mandibles to slightly more than 180 degrees."
Regional Notes
Puerto Rico
Wheeler (1908): Numerous workers and females taken from several colonies nesting under stones in a nearly dry stream bottom behind the Coamo baths. The rediscovery of the female of this species shows that Emery was right in his contention that Roger had described two very different species under the name of Pyrmaica gundlachi (=Strumigenys gundlachi). The females among my specimens agree perfectly with Roger's description and figure.
Florida (USA)
Deyrup (1997) reports this species is often the dominant dacetine ant in moist hammocks and swamp forest.
Clouse (1999) - Found Strumigenys rogeri to be common in wet areas of Everglades National Park and occurring in high densities along marshy trails on the Eastern Florida coast.
Deyrup, Davis & Cover (2000): This species is the common dacetine in bayheads, baygalls, and other swamp forest habitats in south and central Florida. Samples collected by Walter Suter between 1960 and 1970 suggest that several species of Strumigenys, including Strumigenys louisianae, were much more common in the absence of S. rogeri than they are today, although our survey methods may not replicate Suter's, and there have been many other changes in Florida since 1970. Since this species is apparently moving northward, it might be useful to do some intensive sampling ahead of its advance.
Costa Rica
Longino (Ants of Costa Rica) - At La Selva Biological Station, rogeri occurs in leaf litter deep within mature rainforest. Thus, it appears to be one of the few exotics that can invade mature forest, rather than being restricted to synanthropic habitats.
Hong Kong
Using Malaise traps at various locations, Tang et al. (2019) collected female alates that had flown from their nests in sampling conducted from mid-June to mid-July.
Morocco
This species was captured in a natural habitat, 100 m from the road, in the largest cork oak forest in the world at Khémisset, Morocco (Taheri & Reyes-Lopez, 2023).
Castes
Worker
   
| |
| . | Owned by Museum of Comparative Zoology. |
Images from AntWeb
   
| |
| Worker. Specimen code casent0060341. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code casent0064817. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Syntype of Strumigenys rogeri. Worker. Specimen code casent0102080. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by MSNG, Genoa, Italy. |
   
| |
| Worker. Specimen code casent0104529. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
   
| |
| Worker. Specimen code casent0135259. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code casent0178460. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by BNM, Koror, Palau. |
  
| |
| Worker. Specimen code casent0179508. Photographer Erin Prado, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
  
| |
| Worker. Specimen code jtlc000005597. Photographer D. J. Cox, uploaded by California Academy of Sciences. | Owned by JTLC. |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0006035. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Queen (alate/dealate). Specimen code casent0060425. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Queen (alate/dealate). Specimen code casent0145858. Photographer Erin Prado, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Queen (alate/dealate). Specimen code casent0125113. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
    
| |
| Queen (alate/dealate). Specimen code casent0104530. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Male
Images from AntWeb
    
| |
| Male (alate). Specimen code casent0137483. Photographer Erin Prado, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- rogeri. Strumigenys rogeri Emery, 1890b: 68, pl. 7, fig. 6 (w.) ANTILLES. Forel, 1893g: 378 (q.). Senior synonym of incisa: Donisthorpe, 1915d: 341; of sulfurea: Brown, 1954k: 20. See also: Bolton, 1983: 387; Bolton, 2000: 604.
- incisa. Strumigenys incisa Godfrey, 1907: 102 (w.) GREAT BRITAIN. Junior synonym of rogeri: Donisthorpe, 1915d: 341.
- sulfurea. Strumigenys sulfurea Santschi, 1915c: 261 (w.) GABON. Junior synonym of rogeri: Brown, 1954k: 20.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Bolton (1983) - TL 2.3-2.8, HL 0.58-0.74, HW 0.42-0.52, CI 69-75, ML 0.31-0.40, MI 51-58, SL 0.36-0.46, SI 82-89, PW 0.27-0.32, AL 0.58-0.68 (40 measured).
Mandibular blades almost straight and at full closure nearly parallel, not obviously bowed outwards. Apical fork of each blade with 2 spiniform teeth, without intercalary teeth or denticles. Preapical armament of 2 teeth on each blade, set in the distal third of the blade's length; the proximal preapical teeth larger than the distals. Upper scrobe margins narrowly concave immediately behind the frontal lobes, with a pinched-in appearance in full-face view. Behind this the upper scrobe margins feebly divergent to the level of the eye and relatively close together, sometimes even shallowly concave directly above the eye, then developed and the anterior portion of the eye detached from the side of the head. Preocular notch continued onto the ventral surface of the head as a broad impression which runs transversely immediately in front of the level of the eye, but not reaching the ventral midline. Antennal scapes long and slender, approximately straight, the leading edges equipped with a row of narrowly spatulate hairs which are angled towards the apex. Dorsum of head with short narrowly spatulate ground-pilosity which is directed anteriorly, the upper scrobe margins with a row of larger anteriorly curved spoon-shaped hairs. With the head in profile the dorsum with 6 standing hairs which are arranged as a row of 4 transversely close to the occipital margin and a more anteriorly situated pair. Dorsum of head reticulate-punctate. Pronotal humeri each with a long fine flagellate hair and the mesonotum with a single pair of stout standing hairs. Otherwise the dorsal alitrunk without standing hairs, the ground-pilosity of sparse narrow hairs which are closely applied to the surface. With the alitrunk in profile the posterior portion of the mesonotum sharply depressed, the metanotal groove represented by a transverse line across the dorsum but not or only minutely impressed. Propodeal teeth triangular and subtended by narrow infradental lamellae. Sides of alitrunk sometimes completely smooth but usually the propodeum punctulate and the pronotum with faint traces of striolate or costulate sculpture anteriorly. Pronotal dorsum longitudinally striolate or costulate on a finely punctate surface, but in some the costulae may be very feeble and indistinct; the median costula is usually stronger and more sharply defined than any other and in many samples forms a weak median longitudinal carina at least on the: anterior half of the pronotum. Remainder of dorsal alitrunk reticulate-punctate. Dorsum of petiole node weakly reticulate-punctate, the postpetiole generally smooth but sometimes with vague sculptural vestiges. Petiole in profile with a spongiform ventral strip and the node with a transverse collar posteriorly. In profile the postpetiole with large ventral and lateral spongiform lobes. In dorsal view the postpetiole with a posterior spongiform strip which abuts a similar but narrower strip on the base of the first tergite. Basigastral costulae sparse but sharply defined. Dorsal surfaces of petiole, postpetiole and gaster with stout standing hairs which are weakly swollen apically. Colour dull yellow to light medium brown.
Type Material
Bolton (2000):
Holotype worker, ST THOMAS I. (West Indies) (Museo Civico di Storia Naturale, Genoa) [examined].
Strumigenys incisa Godfrey, 1907: 102 [attributed to Forel]. Syntype workers, GREAT BRITAIN: Scotland, Edinburgh, hothouse in Royal Botanic Garden, 10.vi.1904 (R. Godfrey) (The Natural History Museum) [examined].
Strumigenys sulfurea Santschi, 1915: 261. Syntype workers, GABON: Samkita (F. Faure) (Naturhistorisches Museum, Basel) [examined].
References
- Bharti, H. & Akbar, S.A. 2013. Taxonomic studies on the ant genus Strumigenys Smith, 1860 (Hymenoptera, Formicidae) with report of two new species and five new records including a tramp species from India. Sociobiology 60, 387-396 (doi:10.13102/sociobiology.v60i4.387-396).
- Blard, F., Dorow, W.-H.-O., Delabie, J. H. C. 2003. Les Fourmis de l’île de la Réunion (Hymenoptera, Formicidae). Bulletin de La Société Entomologique de France, 108(2), 127–137 (doi:10.3406/bsef.2003.16939).
- Bolton, B. 1983. The Afrotropical dacetine ants (Formicidae). Bulletin of the British Museum (Natural History). Entomology. 46:267-416. (page 387, redescription of worker)
- Bolton, B. 2000. The ant tribe Dacetini. Memoirs of the American Entomological Institute. 65:1-1028. (page 604, catalogue)
- Borowiec, L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Brown, W. L., Jr. 1954k. The ant genus Strumigenys Fred. Smith in the Ethiopian and Malagasy regions. Bulletin of the Museum of Comparative Zoology 112: 1-34 (page 20, senior synonym of sulfurea)
- Brown, W. L., Jr. 1962c. The neotropical species of the ant genus Strumigenys Fr. Smith: synopsis and keys to the species. Psyche. 69:238-267.
- Clouse, R. 1999: Leaf-litter inhabitants of a Brazilian pepper stand in Everglades National Park. Florida Entomologist 82: 388-403.
- Dekoninck, W., Wauters, N., Delsinne, T. 2019. Capitulo 35. Hormigas invasoras en Colombia. Hormigas de Colombia.
- Deyrup, M. 1997. Dacetine ants of the Bahamas (Hymenoptera: Formicidae). Bahamas J. Sci. 5:2-6.
- Deyrup, M., Davis, L. & Cover, S. 2000. Exotic ants in Florida. Transactions of the American Entomological Society 126, 293-325.
- Deyrup, M.; Trager, J. 1985 [1984]. Strumigenys rogeri, an African dacetine ant new to the U.S. (Hymenoptera: Formicidae). Fla. Entomol. 67: 512-516 (page 512, record in United States)
- Donisthorpe, H. 1915f. British ants, their life-history and classification. Plymouth: Brendon & Son Ltd., xv + 379 pp. (page 341, senior synonym of incisa)
- Emery, C. 1890c. Studii sulle formiche della fauna neotropica. Bullettino della Società Entomologica Italiana 22:38-80. (page 68, pl. 7, fig. 6 worker described)
- Fontenla, J.L., Brito, Y.M. 2011. Hormigas invasoras y vagabundas de Cuba. Fitosanidad 15(4), 253-259.
- Forel, A. 1893j. Formicides de l'Antille St. Vincent, récoltées par Mons. H. H. Smith. Trans. Entomol. Soc. Lond. 1893: 333-418 (page 378, queen described)
- Hamer, M.T., Telfer, M.G., Turner, C.R. 2023. Rediscovered after 102 years: Strumigenys rogeri (Formicidae; Myrmicinae) is rediscoverd in Britain from the Eden Project East Cornwall (VC 22). British Journal of Entomology, Natural History 36, 225-231.
- Hamer, M.T., Turner, C.R. 2024. Strumigenys emmae (Emery, 1890) (Myrmicinae) new to Britain, with an updated key to the known Strumigenys of the West Palaearctic. Zootaxa 5415(4), 570–576 (doi:10.11646/zootaxa.5415.4.6).
- Ito, F., Makita, S., Nakao, H., Hosokawa, R., Kikuchi, T., Yamane, S. 2021. Thelytokous parthenogenesis by dealate queens in the myrmicine ant Monomorium hiten distributed in Nansei Islands, western Japan, with description of the male. Asian Myrmecology 14: e014001 (doi:10.20362@am.014001).
- Larabee, F.J., Suarez, A.V. 2014. The evolution and functional morphology of trap-jaw ants (Hymenoptera: Formicidae). Myrmecological News 20: 25-36.
- Lee, C.C., Hsu, S.H., Yang, C.C., Lin, C.C. 2017. Thelytokous parthenogenesis in the exotic dacetine ant Strumigenys rogeri (Hymenoptera: Formicidae) in Taiwan. Entomological Science 21: 28–33.
- Liu, C., Sarnat, E.M., Friedman, N.R., Hita Garcia, F., Darwell, C., Booher, D., Kubota, Y., Mikheyev, A.S., Economo, E.P. 2020. Colonize, radiate, decline: Unraveling the dynamics of island community assembly with Fijian trap‐jaw ants. Evolution 74, 1082–1097 (doi:10.1111/EVO.13983).
- Lubertazzi, D. 2019. The ants of Hispaniola. Bulletin of the Museum of Comparative Zoology, 162(2), 59-210 (doi:10.3099/mcz-43.1).
- MacGown, J.A., Wetterer, J.K. 2013. Distribution and biological notes of Strumigenys margaritae (Hymenoptera: Formicidae: Dacetini). Terrestrial Arthropod Reviews 6, 247–255 (doi:10.1163/18749836-06001066).
- Meurgey, F. 2020. Challenging the Wallacean shortfall: A total assessment of insect diversity on Guadeloupe (French West Indies), a checklist and bibliography. Insecta Mundi 786: 1–183.
- Michlewicz, M. 2022. Strumigenys emmae (Emery, 1890) (Hymenoptera: Formicidae) in Poland – first record of this pantropic ant species from Europe with remarks on its biology. Annals of the Upper Silesian Museum in Bytom, Entomology 31 (online 007): 1-5 (doi:10.5281/ZENODO.6559247).
- Oussalah, N., Marniche, F., Espadaler, X., Biche, M. 2019. Exotic ants from the Maghreb (Hymenoptera, Formicidae) with first report of the hairy alien ant Nylanderia jaegerskioeldi (Mayr) in Algeria. Arxius de Miscel·lània Zoològica, 45–58 (doi:10.32800/amz.2019.17.0045).
- Purkaret, A., Repta, F., Selnekovic, D., Jancik, L., Holecova, M. 2021. Notes on Strumigenys argiola (Emery, 1869) (Hymenoptera: Formicidae) with emphasis on its distribution, ecology and behaviour. Entomofauna Carpathica 33(2): 73-88.
- Rosas-Mejía, M., Guénard, B., Aguilar-Méndez, M. J., Ghilardi, A., Vásquez-Bolaños, M., Economo, E. P., Janda, M. 2021. Alien ants (Hymenoptera: Formicidae) in Mexico: the first database of records. Biological Invasions 23(6), 1669–1680 (doi:10.1007/s10530-020-02423-1).
- Sarnat, E.M., Hita-Garcia, F., Dudley, K., Liu, C., Fischer, G., Economo, E.P. 2019. Ready species one: Exploring the use of augmented reality to enhance systematic biology with a revision of Fijian Strumigenys (Hymenoptera: Formicidae). Insect Systematics and Diversity 3(6): 6; 1–43 (doi:10.1093/isd/ixz005).
- Taheri, A., Reyes-Lopez, J.L. 2023. New and additional records for the ant fauna (Hymenoptera, Formicidae) of Morocco. Journal of the Entomological Research Society 25(1): 1-10 (doi:10.51963/jers.v25i1.1995)
- Tang, K. L., Guénard, B. 2023. Further additions to the knowledge of Strumigenys (Formicidae: Myrmicinae) within South East Asia, with the descriptions of 20 new species. European Journal of Taxonomy 907, 1–144 (doi:10.5852/ejt.2023.907.2327).
- Tang, K.L., Pierce, M.P., Guénard, B. 2019. Review of the genus Strumigenys (Hymenoptera, Formicidae, Myrmicinae) in Hong Kong with the description of three new species and the addition of five native and four introduced species records. ZooKeys 831: 1–48 (DOI 10.3897/zookeys.831.31515).
- Wang, C., Chung, F.-Y., Lin, C.-C., Gibson, J. C., McGuire, S., Suarez, A. V., Billen, J. 2023. The spongiform tissue in Strumigenys ants contains exocrine glands. Arthropod Structure & Development 73, 101246 (doi:10.1016/j.asd.2023.101246).
- Wang, C., Lin, C.-C., Keller, R.A., Billen, J. 2021. The ‘hairwheels’ in Strumigenys ants are not glandular. Asian Myrmecology 13: e013004 (doi:10.20362/am.013004).
- Wang, C., Sung, P.-J., Lin, C.-C., Ito, F., Billen, J. 2023. Parthenogenetic reproduction in Strumigenys ants: An update. Insects 14, 195 (doi:10.3390/insects14020195).
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Wetterer, J.K. 2012. Worldwide spread of Roger's dacetine ant, Strumigenys rogeri (Hymenoptera: Formicidae). Myrmecological News 16:1-6.
- Wetterer, J.K. 2021. Ants (Hymenoptera, Formicidae) of St. Vincent, West Indies. Sociobiology 68, e6725 (doi:10.13102/sociobiology.v68i2.6725).
- Wheeler, W. M. 1908a. The ants of Porto Rico and the Virgin Islands. Bull. Am. Mus. Nat. Hist. 24: 117-158.
References based on Global Ant Biodiversity Informatics
- Belshaw R., and B. Bolton. 1994. A survey of the leaf litter ant fauna in Ghana, West Africa (Hymenoptera: Formicidae). Journal of Hymenoptera Research. 3: 5-16.
- Bolton B. 1983. The Afrotropical dacetine ants (Formicidae). Bulletin of the British Museum (Natural History). Entomology 46: 267-416.
- Bolton, B. 2000. The Ant Tribe Dacetini. Memoirs of the American Entomological Institute 65
- Brown W. L. Jr. 1954. The ant genus Strumigenys Fred. Smith in the Ethiopian and Malagasy regions. Bulletin of the Museum of Comparative Zoology 112: 1-34.
- IZIKO South Africa Museum Collection
- Santschi F. 1915. Nouvelles fourmis d'Afrique. Annales de la Société Entomologique de France 84: 244-282.
- Taylor B. 1979. Ants of the Nigerian Forest Zone (Hymenoptera: Formicidae). III. Myrmicinae (Cardiocondylini to Meranoplini). Cocoa Research Institute of Nigeria Research Bulletin 6: 1-65.
- Yeo K., and A. Hormenyo. 2007. A Rapid Survey of Ants in Ajenjua Bepo and Mamang River Forest Reserves, Eastern Region of Ghana. Pp 27-29. In McCullough, J., P. Hoke, P. Naskrecki, and Y. Osei-Owusu (eds.). 2008. A Rapid Biological Assessment of the Ajenjua Bepo and Mamang River Forest Reserves, Ghana. RAP Bulletin of Biological Assessment 50. Conservation International, Arlington, VA, USA.



