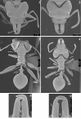
Daceton armigerum
| Daceton armigerum | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Daceton |
| Species: | D. armigerum |
| Binomial name | |
| Daceton armigerum (Latreille, 1802) | |
| Synonyms | |
Daceton armigerum has been studied extensively by Wheeler & Wheeler (1954, description of larvae), Wilson (1962, ecology and behavior), Blum & Portocarrero (1966, trail pheromone and venom), Hölldobler et al. (1990, chemical communication), Moffet & Tobin (1991, physical castes), Groenenberg (1996, mandibular mode of action), and Bolton (1999, 2000, classification).
Photo Gallery
Identification
Key to the two Daceton species.
Pronotal humeral spines bifurcate, the anterior tip larger than the posterior one. First gastral tergite without standing hairs (sometimes with very short, appressed hairs). Mesonotum and metanotum divided by a strongly impressed metanotal groove. Inner (masticatory) margin of mandibles with a row of short think setae . . . . . Daceton armigerum
Pronotal humeral spines long and simple. First gastral tergite with suberect to subdecumbent hairs. Mesonotum and metanotum divided by a weakly impressed metanotal groove. Inner (masticatory) margin of mandibles lacking a row of short thick setae . . . . . Daceton boltoni
- character differences
Keys including this Species
Distribution
Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname, Trinidad and Venezuela.
Latitudinal Distribution Pattern
Latitudinal Range: 8.75° to -14.5°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Bolivia, Brazil (type locality), Ecuador, French Guiana, Guyana, Peru, Suriname, Trinidad and Tobago, Venezuela.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Habitat
Terra Firma and flooded forests.
Biology
Azorsa & Sosa (2008) - Typically nests in cavities in the branches and trunks of trees previously bored by beetles and other insects. Blum and Portocarrero (1966) and Moffet and Tobin (1991) state that colonies of D. armigerum contain up to 2500 individuals, whereas Wilson (1962) and Hölldobler & Wilson (1990) estimate that colonies contain between 5000 to 10000 workers. Daceton armigerum has a complex continuously polymorphic caste system, in which smaller workers nurse the brood and larger workers hunt, dismember prey items, and defend the nest (Wilson 1962; Oster & Wilson 1978; Hölldobler & Wilson 1990). Wilson (1962) reports that workers of this highly predaceous myrmicine ant hunt individually for a variety of live insects, including flies, grasshoppers, larvae and adults of moths and beetles, and fulgorids. In addition, some workers have been observed tending coccids (Bodkin in Crawley 1916; Brown & Wilson 1960; Wilson 1962). (Refer to Wilson [1962] for further information on the behavior of D. armigerum.) Recently, Yanoviak et al. (2005) found that individuals of D. armigerum show controlled aerial descent behavior. The genus has been considered primitive with respect to other members of the Dacetini (Brown and Wilson 1959; Bolton 1998, 1999, 2000), but a phylogenetic analysis of the tribe is necessary to fully understand the relationships of its constituent species and genera. Current molecular phylogenetic evidence suggests that Dacetini may not be monophyletic (Brady et al. 2006).
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Diptera
- This species is a host for the phorid fly Apocephalus sp. (a parasite) (Brown et al., 2015) (injured).
Fungi
- This species is a host for the fungus Ophiocordyceps daceti (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
- This species is a host for the fungus Ophiocordyceps daceti (a pathogen) (Araujo et al., 2018).
Life History Traits
- Mean colony size: 10,000 (Blum & Portocarrero, 1965; Beckers et al., 1989)
- Foraging behaviour: solitary forager (Blum & Portocarrero, 1965; Beckers et al., 1989)
Castes
Worker
Daceton X-ray micro-CT scan 3D model of Daceton armigerum (worker) prepared by the Economo lab at OIST.
X-ray micro-CT scan 3D model of Daceton armigerum (worker) prepared by the Economo lab at OIST.
Head of specialised, arboreal trap-jaw ant from South America. See on Sketchfab. See list of 3D images.
Male
Images from AntWeb
      
| |
| Male (alate). Specimen code casent0178490. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by MZSP, Sao Paulo, Brazil. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- armigerum. Formica armigera Latreille, 1802c: 244, pl. 9, fig. 58 (w.) (no state data).
- Type-material: syntype (?) workers (number not stated).
- Type-locality: none given (“collection du Stathouder”).
- Type-depository: unknown (perhaps MNHN).
- Smith, F. 1853: 226 (q.m.); Wheeler, G.C. & Wheeler, J. 1955a: 122 (l.).
- Combination in Formica (Atta): Guérin-Méneville, 1844a: 421;
- combination in Daceton: Perty, 1833: 136; Smith, F. 1853: 226.
- Status as species: Perty, 1833: 136; Guérin-Méneville, 1844a: 421; Smith, F. 1853: 226; Smith, F. 1858b: 160; Roger, 1862c: 290; Roger, 1863b: 40; Mayr, 1884: 38; Mayr, 1886c: 360; Dalla Torre, 1893: 149; Emery, 1894c: 140; Forel, 1895b: 136; Forel, 1907e: 3; Crawley, 1916b: 372; Mann, 1916: 452; Wheeler, W.M. 1916c: 9; Wheeler, W.M. 1923a: 4; Emery, 1924d: 316; Borgmeier, 1927c: 120; Borgmeier, 1934: 103; Kempf, 1961b: 514; Wilson, 1962b: 403; Kempf, 1970b: 335; Kempf, 1972a: 95; Bolton, 1995b: 168; Gronenberg, 1996: 2012; Bolton, 1999: 1655; Bolton, 2000: 18; Azorsa & Sosa-Calvo, 2008: 30; Sosa-Calvo, et al. 2010: 39 (in key); Bezděčková, et al. 2015: 117; Fernández & Serna, 2019: 851.
- Senior synonym of cordata: Roger, 1862c: 290; Mayr, 1863: 406; Roger, 1863b: 40; Dalla Torre, 1893: 149; Forel, 1895b: 136; Emery, 1924d: 317; Borgmeier, 1927c: 120; Kempf, 1972a: 95; Bolton, 1995b: 168.
- [Note: Mayr, 1863: 406, gives cordata as senior synonym, but armigerum has priority (Roger, 1863b: 40).]
- Distribution: Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname, Trinidad, Venezuela.
- cordata. Myrmecia cordata Fabricius, 1804: 425 (w.) “South America”.
- Type-material: 3 syntype workers.
- [Note: Zimsen, 1964: 428, cites 3w syntypes (2 ZMUC, 1 ZMUK).]
- Type-locality: South America: (“Habitat in America meridionali Dom. Smidt. Mus. Dom. de Sehestedt.”) (no further data).
- [Note: type-material is labeled “Essequibo Smidt. Mus. de Sehestedt”; hence type-locality is Guyana: Essequibo.].
- Type-depositories: ZMUC, ZMUK.
- Junior synonym of armigerum: Roger, 1862c: 290; Mayr, 1863: 406; Roger, 1863b: 40; Dalla Torre, 1893: 149; Forel, 1895b: 136; Emery, 1924d: 317; Borgmeier, 1927c: 120; Kempf, 1972a: 95; Bolton, 1995b: 168.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Azorsa & Sosa (2008) - Measurements (mm): EL 0.44–0.87, GL 1.89–4.50, HL 1.44–4.06, HW 1.58–4.17, ML 0.79–3.32, PL 0.78–1.93, PPL 0.26-0.51, PSL 0.32-1.59, PW 1.38-4.60, SL 0.94-2.77, TL 6.91-17.8, WL 1.70–4.24.
Indexes: CI 102–113, MI 55–88, PI 41–52, PSI 18–39, SI 59–73 (17 measured).
Polymorphic. Head wider than long, heart-shaped. Mandibles linear and elongate, each with an apical fork of two teeth that overlap at full closure, of which the ventral tooth is the largest. Inner (masticatory) margin of mandibles lacking any dentition but with a series of short, thick setae that differ from any other pilosity present on mandibles. Outer margin of mandibles lacking hairs completely or with very short, appressed hairs. Mandibles, in full-face view, somewhat long and narrow [(MW/ML')*100= 25–40]. Palp formula 5,3. Depressions, adjacent to and ventral to the mandibular insertion, deep. Clypeus without standing hairs. Pronotal humeri with acute tubercles. Lateral pronotal spines bifurcate, the anterior tips larger than the posterior ones. Metanotal groove deeply impressed. Mesosoma glabrous. Petiolar node with an anterior-lateral pair of long spines and a pair of small but sharp tubercles, located underneath the anterior-lateral spines. First gastral tergite finely reticulate and devoid of any erect or semi-erect pilosity, sometimes with very short, appressed hairs. Color of head, mesosoma, and metasoma, usually red-brown to red-yellowish, sometimes dark red-brown or rarely bicolored; petiole, postpetiole, and gaster darker than rest of body.
Most of the within-species morphological variation in D. armigerum workers is manifested in the form of the promesonotum and, to a lesser degree, in the forms of the petiole, postpetiole, and gaster. This variation includes: (i) Lateral spines bifurcate, the posterior spine projecting upwards and curving at the tip in major workers, whereas in small or median workers this spine not curving at the tip. In small workers the posterior spine is very short, almost vestigial when viewed in profile, but conspicuous in dorsal view. (ii) Short, simple, and appressed hairs present on the first gastral tergite in some individuals from Brazil and Peru. On other workers, hairs on the first gastral segment are absent. (iii) Humeral spines, in smaller workers, vestigial or present as very low carinae. Median and larger-sized workers with humeral tubercles that are spinose or acute. (iv) The posterior pair of petiolar tubercles reduced, rounded and low in smaller workers, whereas tubercles acute in larger workers. (v) Large workers with posterior promesonotal tubercles truncate and flattened in profile. (vi) Anterior spines of petiole long and diverging with intervening space concave or with intervening space discontinuous. Anterior spines of petiole in smaller workers shorter than in other castes.
Type Material
Azorsa & Sosa (2008): Syntype(?) worker, Brazil (not seen).
Myrmecia cordata. Syntypes, 2 workers, America Meridionali [South America]. (seen)
Myrmecia cordata Fabricius, Syntypes, 2 workers, labeled: “Essequibo [possibly Guyana], Smidt. Mus. de Sehestedt. Armigerum, Latr. [Latreille] Myrmecia cordata, worker, Fabr. [Fabricius].” Deposited in Zoologisk Museum, University of Copenhagen.
References
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Als, V., Narendra, A., Arthofer, W., Krapf, P., Steiner, F.M., Schlick-Steiner, B.C. 2021. Colony structure, population structure, and sharing of foraging trees in the ant Myrmecia nigriceps (Hymenoptera: Formicidae). Insectes Sociaux 68, 327–335 (doi:10.1007/s00040-021-00831-7).
- Araújo, J.P.M., Evans, H.C., Kepler, R., Hughes, D.P. 2018. Zombie-ant fungi across continents: 15 new species and new combinations within Ophiocordyceps. I. Myrmecophilous hirsutelloid species. Studies in Mycology 90: 119–160 (DOI 10.1016/j.simyco.2017.12.002).
- Beckers R., Goss, S., Deneubourg, J.L., Pasteels, J.M. 1989. Colony size, communication and ant foraging Strategy. Psyche 96: 239-256 (doi:10.1155/1989/94279).
- Billen, J., Al-Khalifa, M.S., Silva, R.R. 2017. Pretarsus structure in relation to climbing ability in the ants Brachyponera sennaarensis and Daceton armigerum. Saudi Journal of Biological Sciences 24, 830–836 (doi:10.1016/j.sjbs.2016.06.007).
- Billen, J.P.J. 2019. Diversidad y morfología de las glándulas exocrinas en las hormigas. Pp. 165-174 in: Fernández, F., Guerrero, R.J., Delsinne, T. (eds.) 2019d. Hormigas de Colombia. Bogotá: Universidad Nacional de Colombia, 1198 pp.
- Blum, M. S.; Portocarrero, C. A. 1964. Chemical releasers of social behavior - IV. The hindgut as the source of the odor trail pheromone in the Neotropical army ant genus Eciton. Ann. Entomol. Soc. Am. 57: 793-794 (behavior)
- Bolton, B. 1995b. A new general catalogue of the ants of the world. Cambridge, Mass.: Harvard University Press, 504 pp. (page 168, catalogue)
- Bolton, B. 1999. Ant genera of the tribe Dacetonini (Hymenoptera: Formicidae). J. Nat. Hist. 3 33: 1639-1689 (page 1655, see also)
- Brown, W. L., Jr. 1988h. Data on Malpighian tubule numbers in ants (Hymenoptera: Formicidae). Pp. 17-27 in: Trager, J. C. (ed.) Advances in myrmecology. Leiden: E. J. Brill, xxvii + 551 pp. (page 23, anatomy)
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Cantone S. 2018. Winged Ants, The queen. Dichotomous key to genera of winged female ants in the World. The Wings of Ants: morphological and systematic relationships (self-published).
- Cardoso Neto, J.A., Leal, L.C., Baccaro, F.B. 2019. Temporal and spatial gradients of humidity shape the occurrence and the behavioral manipulation of ants infected by entomopathogenic fungi in Central Amazon. Fungal Ecology 42, 100871 (doi:10.1016/j.funeco.2019.100871).
- Dejean, A., Compin, A., Delabie, J.H.C., Azémar, F., Corbara, B., Leponce, M. 2019. Biotic and abiotic determinants of the formation of ant mosaics in primary Neotropical rainforests. Ecological Entomology 44, 560–570 (doi:10.1111/een.12735).
- Delsinne, T., Sonet, G., Arias-Penna, T.M. 2019. Capitulo 21. Subfamilia Paraponerinae. Hormigas de Colombia.
- Diller, E. 1990. Die von Spix und Martius 1817-1820 in Brasilien gesammelten und von J.A.M. Perty 1833 bearbeiteten Hymenopteren in der Zoologischen Staatssammlung München. Spixiana 13 (1): 61-81.
- Fernández, F.; Palacio, E. E.; Mackay, W. P.; Mackay, E. S. 1996. Introducción al estudio de las hormigas (Hymenoptera: Formicidae) de Colombia. Pp. 349-412 in: Andrade, M. G., Amat García, G., Fernández, F. (eds.) Insectos de Colombia. Estudios escogido (page 381, anatomy)
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Gronenberg, W. 1996. The trap-jaw mechanism in the dacetine ants Daceton armigerum and Strumigenys sp. J. Exp. Biol. 1 199: 2021-2033 (page 2021, anatomy)
- Hölldobler, B.; Palmer, J. M.; Moffett, M. W. 1990. Chemical communication in the dacetine ant Daceton armigerum (Hymenoptera: Formicidae). J. Chem. Ecol. 1 16: 1207-1219 (biology)
- Jansen, G., Savolainen, R. 2010. Molecular phylogeny of the ant tribe Myrmicini (Hymenoptera: Formicidae). Zoological Journal of the Linnean Society 160(3), 482–495 (doi:10.1111/j.1096-3642.2009.00604.x).
- Kempf, W. W. 1961b. A survey of the ants of the soil fauna in Surinam (Hymenoptera: Formicidae). Stud. Entomol. 4: 481-524 (page 514, behavior)
- Kempf, W. W. 1972b. Catálogo abreviado das formigas da regia~o Neotropical. Stud. Entomol. 15: 3-344 (page 95, catalogue)
- Larabee, F.J., Suarez, A.V. 2014. The evolution and functional morphology of trap-jaw ants (Hymenoptera: Formicidae). Myrmecological News 20: 25-36.
- Latreille, P.A. 1802. Histoire naturelle des fourmis, et recueil de mémoires et d'observations sur les abeilles, les araignées, les faucheurs, et autres insectes. Paris: Impr. Crapelet (chez T. Barrois), xvi + 445 pp.
- Perty, M. 1833. Delectus animalium articulatorum, quae in itinere per Brasiliam annis MDCCCXVII-MDCCCXX jussu et auspiciis Maximiliani Josephi I. Bavariae regis augustissimi peracto, collegerunt Dr. J. B. Spix et Dr. C. F. Ph. de Martius. Fasc. 3. Monach (page 136, Combination in Daceton)
- Przybyszewski, K.R., Silva, R.J., Vicente, R.E., Garcia Freitas, J.V., Pereira, M.J.B., Izzo, T.J., Tonon, D.S. 2020. Can baited pitfall traps for sampling dung beetles replace conventional traps for sampling ants? Sociobiology 67, 376-387 (doi:10.13102/sociobiology.v67i3.5201).
- Ramalho, M.de O., Martins, C., Morini, M.S.C., Bueno, O.C. 2020. What can the bacterial community of Atta sexdens (Linnaeus, 1758) tell us about the habitats in which this ant species evolves? Insects 11, 332. (doi:10.3390/INSECTS11060332).
- Ramalho, M.O., Duplais, C., Orivel, J., Dejean, A., Gibson, J.C., Suarez, A.V., Moreau, C.S. 2020. Development but not diet alters microbial communities in the Neotropical arboreal trap jaw ant Daceton armigerum: an exploratory study. Scientific Reports 10, 7350 (doi:10.1038/s41598-020-64393-7).
- Roger, J. 1862c. Synonymische Bemerkungen. 1. Ueber Formiciden. Berl. Entomol. Z. 6: 283-297 (page 290, senior synonym of cordata)
- Smith, F. 1853 [1854]. Monograph of the genus Cryptocerus, belonging to the group Cryptoceridae - family Myrmicidae - division Hymenoptera Heterogyna. Trans. Entomol. Soc. Lond. (2) 2: 213-228 (page 226, queen, male described)
- Wheeler, G. C.; Wheeler, J. 1955a [1954]. The ant larvae of the myrmicine tribes Basicerotini and Dacetini. Psyche (Camb.) 61: 111-145 (page 122, larva described)
- Wheeler, G. C.; Wheeler, J. 1973c. The ant larvae of the tribes Basicerotini and Dacetini: second supplement (Hymenoptera: Formicidae: Myrmicinae). Pan-Pac. Entomol. 49: 207-214 (page 211, larva described)
- Wilson, E. O. 1962b. Behavior of Daceton armigerum (Latreille), with a classification of self-grooming movements in ants. Bulletin of the Museum of Comparative Zoology 127: 403-421 (page 401, behavior)
- Yanoviak, S.P., Frederick, D.N. 2014. Water surface locomotion in tropical canopy ants. Journal of Experimental Biology 217, 2163–2170 (doi:10.1242/jeb.101600).
References based on Global Ant Biodiversity Informatics
- Alonso L. E. 2010. A preliminary survey of the ants of the Kwamalasamutu region, SW Suriname. In: OShea, B.J., L.E. Alonso, & T.H. Larsen, (eds.). 2011. A Rapid Biological Assessment of the Kwamalasamutu region, Southwestern Suriname. RAP Bulletin of Biological Assessment 63. Conservation International, Arlington, VA.
- Alonso L. E., J. Persaud, and A. Williams. 2016. Biodiversity assessment survey of the south Rupununi Savannah, Guyana. BAT Survey Report No.1, 306 pages.
- Alonso L. E., and J. A. Helms. 2013. A Rapid Assessment of the Ants of the Grensgebergte and Kasikasima Regions of Southeastern Suriname. A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname: 109-118.
- Azorsa F. and J. Sosa-Calvo. 2008. Description of a remarkable new species of ant in the genus Daceton Perty (Formicidae: Dacetini) from South America. Zootaxa 1749: 27-38
- Dejean A., A. Compin, J. H. C. Delabie, F. Azemar, B. Corbara, and M. Leponce. 2019. Biotic and abiotic determinants of the formation of ant mosaics in primary Neotropical rainforests. Ecological Entomology https://doi-org.eproxy.lib.hku.hk/10.1111/een.12735
- Dejean A., J. H. C. Delabie, B. Corbara, F. Azemar, S. Groc, et al. 2012. The Ecology and Feeding Habits of the Arboreal Trap-Jawed Ant Daceton armigerum. PLoS ONE 7(6): e37683. doi:10.1371/journal.pone.0037683
- Fernandez F. C., and L. Schneider S. 1989. Reconocimiento de hormigas en la reserva La Macarena. Revista Colombiana de Entomologia 15(1): 38-44.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Field Museum Collection, Chicago, Illinois (C. Moreau)
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Galvis J. P., and F. Fernandez. 2007. SINOPSIS DE LAS HORMIGAS DACETINAS DE LOS GÉNEROS Daceton perty Y Acanthognathus mayr (FORMICIDAE: MYRMICINAE) DE COLOMBIA. Acta biol. Colomb. 12 (1): 123.
- Herzog H., M. C. Muller, M. Granier, J. Lattke, and K. Jaffe. 1989. Notas etnomirmecologicas Yanomani. Acta Terramaris 1: 51-57.
- Jaffe, K., et al. 2007. Comparing the ant fauna in a tropical and a temperat forest canopy. Ecotropicos 20(2):74-81
- Kempf W. W. 1961. A survey of the ants of the soil fauna in Surinam (Hymenoptera: Formicidae). Studia Entomologica 4: 481-524.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Miranda P. N., F. B. Baccaro, E. F. Morato, M. A. Oliveira. J. H. C. Delabie. 2017. Limited effects of low-intensity forest management on ant assemblages in southwestern Amazonian forests. Biodivers. Conserv. DOI 10.1007/s10531-017-1368-y
- Pires de Prado L., R. M. Feitosa, S. Pinzon Triana, J. A. Munoz Gutierrez, G. X. Rousseau, R. Alves Silva, G. M. Siqueira, C. L. Caldas dos Santos, F. Veras Silva, T. Sanches Ranzani da Silva, A. Casadei-Ferreira, R. Rosa da Silva, and J. Andrade-Silva. 2019. An overview of the ant fauna (Hymenoptera: Formicidae) of the state of Maranhao, Brazil. Pap. Avulsos Zool. 59: e20195938.
- Radoszkowsky O. 1884. Fourmis de Cayenne Française. Trudy Russkago Entomologicheskago Obshchestva 18: 30-39.
- Sosa-Calvo J., T. R. Schultz, and J. S. LaPolla. 2010. A review of the dacetine ants of Guyana (Formicidae: Myrmicinae). Journal of Hymenoptera Research 19: 12-43.
- Vasconcelos H. L., J. M. S. Vilhena, W. E. Magnusson, and A. L. K. M. Albernaz. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. Journal of Biogeography 33: 1348-1356.
- Vasconcelos, H.L., J.M.S. Vilhena, W.E. Magnusson and A.L.K.M. Albernaz. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. Journal of Biogeography 33:1348-1356
- Vicente R. E., J. Damborz, and M. Rocha Barreto. 2011. New distribution record of Daceton boltoni Azorsa and Sosa-Calvo, 2008 (Insecta: Hymenoptera) ant in the Brazilian Amazon. Check List 7(6): 878-879.
- Wheeler W. M. 1916. Ants collected in British Guiana by the expedition of the American Museum of Natural History during 1911. Bulletin of the American Museum of Natural History 35: 1-14.
- Wilson E. O. 1962. Behavior of Daceton armigerum (Latreille), with a classification of self-grooming movements in ants. Bulletin of the Museum of Comparative Zoology 127: 403-421.
- Wilson, E.O. 1987. The Arboreal Ant Fauna of Peruvian Amazon Forests: A First Assessment. Biotropica 19(3):245-251.
- da Silva Monteiro D., R. E. Vicente, J. de Oliveira, and T. Junqueira Izzo. 2011. Composicao e riqueza de Formigas (Hymenoptera: Formicidae) em areas de floresta ombrofila densa e reflorestamento de Teca (Tectona grandis L. F. - Verbenaceae) na Fazenda Sao Nicolau, Cotriguacu, MT. Chapter 13, pp 287-301. In: Descobrindo a Amazonia Meridional: Biodiversidade da Fazenda Sao Nicolau; de Jesus Rodrigues D., T. J. Izoo, and L. D. Battirola (Eds).
- da Silva de Oliveira A. B., and F. A. Schmidt. 2019. Ant assemblages of Brazil nut trees Bertholletia excelsa in forest and pasture habitats in the Southwestern Brazilian Amazon. Biodiversity and Conservation 28(2): 329-344.