Crematogaster carinata
| Crematogaster carinata | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Crematogastrini |
| Genus: | Crematogaster |
| Species: | C. carinata |
| Binomial name | |
| Crematogaster carinata Mayr, 1862 | |
| Synonyms | |
| |
This is a wide ranging, variable ant. It may in fact be a complex of closely related species that prove to be ecological distinct as opposed to having clearly separable morphological differences. In some areas C. carinata (see details in the biology section below) form sprawling, polydomous colonies with many queens while in other parts of their range they can have much smaller colonies and a single queen. This species can also be found in parabiotic associations with other arboreal ant species.
| At a Glance | • Polygynous • Xenobiotic • Ant garden |
Identification
Longino (2003) - Costa Rican species in the limata complex are Crematogaster brasiliensis, Crematogaster carinata, Crematogaster limata, and Crematogaster tenuicula. All have abundant erect flexuous setae on the face, moderate length to short posteriorly directed propodeal spines, and elongate tapering petioles. The four species can be difficult to separate. They differ primarily in the nature of the ventral processes of the petiole and postpetiole. Crematogaster carinata has a squared-off anteroventral petiolar process and no postpetiolar process. Both brasiliensis and tenuicula have ventral postpetiolar processes. Crematogaster limata usually lacks a petiolar process but can be very like carinata in some cases. Crematogaster limata is a larger ant with longer propodeal spines. Crematogaster carinata may also be confused with Crematogaster foliocrypta, but foliocrypta has appressed rather than erect tibial pilosity.
Within carinata, there is pronounced variation in the degree of anterior narrowing of the petiole in side view, strength of pronotal carinulae, size of propodeal spines, and some details of queen size and petiole shape. Crematogaster levior is more likely to be a “good” species, in terms of phylogeny and/or genetic similarity. Crematogaster carinata, with its greater geographic range and morphological variability, is more likely to be a set of allopatric or parapatric species, or even a set of broadly sympatric cryptic species.
Crematogaster carinata is also very similar to the South American species Crematogaster levior. The two species are not cleanly separable on morphological grounds. In this revision I have restricted C. levior to a specialized parabiotic associate of Camponotus femoratus. Crematogaster levior and Camponotus femoratus inhabit ant gardens throughout Amazonian South America. All Crematogaster material collected from these ant gardens has the worker pronotum completely smooth and shining. In contrast, I have identified as carinata all material with longitudinal carinulae on the pronotum. However, on purely morphological grounds there is continuous variation from strongly carinate forms, to forms with faint traces of carinulae, to the completely smooth pronotum of levior. Crematogaster levior is polygynous and polydomous like some populations of carinata. Crematogaster levior is always parabiotic with Camponotus femoratus, while C. carinata is facultatively parabiotic with other large ants in the genera Dolichoderus and Odontomachus.
Keys including this Species
Distribution
Longino (2003) - Costa Rica to Brazil (Rio de Janeiro), Bolivia.
Latitudinal Distribution Pattern
Latitudinal Range: 15.6864989° to -64.23°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Barbados, Bolivia, Brazil (type locality), Colombia, Costa Rica, Ecuador, French Guiana, Guatemala, Honduras, Lesser Antilles, Mexico, Panama, Peru, Suriname, Trinidad and Tobago, Venezuela.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
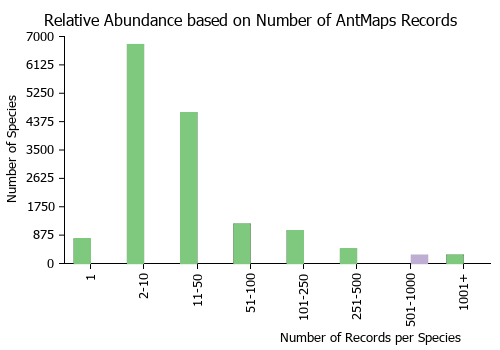
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
|
Biology
Longino (2003) - Crematogaster carinata is a biologically complex species and there is almost certainly some degree of genetic structuring that is not readily revealed by external morphology. This species and its close relative Crematogaster levior often have large polygynous colonies that blanket large sections of forest, with various degrees of mutual foraging and nesting with other unrelated ant species. Forel observed this species nesting with Dolichoderus debilis in Colombia, and coined the term parabiosis for the mutual sharing of nest space and foraging columns by multiple species (Forel 1898). Further examples of parabiosis involving carinata (as parabiotica) were described by Wheeler (1921a).
In the Atlantic lowland forests of northeastern Costa Rica, including coastal strand vegetation, C. carinata forms large polygynous, perhaps even unicolonial colonies. Columns of workers are spread over second growth vegetation and multiple crowns of trees, with small clusters of physogastric queens, brood, and workers dispersed in dead twigs and branches and under epiphytes. There is no obvious colony center, and it is difficult to discern colony boundaries. At La Selva Biological Station it is one of the most common species in the canopy. It occurred in 24 of 52 canopy fogging events. In an old treefall I once found a small aroid with a carinata nest in and around the root ball. The ants had covered the roots with a mass of carton material to form a nest volume about the size of a large orange. Inside were abundant workers, brood, and 43 dealate queens. Some of these queens had torn remnants of wings, suggesting they never left the nest for a nuptial flight and perhaps never mated. In general when multiple queens are found in nests they are evenly distributed in the nest volume, individually or in pairs. Brood is segregated by size. I observed a similar occurrence of large polygynous colonies in the Santa Marta region of Colombia, the same site as Forel's original observations of parabiosis. In contrast to these observations of polygyny, discrete nests with single physogastric queens seem to be the rule in the Pacific and southeastern Atlantic lowlands of Costa Rica, and at some sites in Venezuela.
On occasion I find aggregations of workers only, with no brood. All the dead sticks in a patch of forest understory will be filled with workers. The nest aggregation will be bounded, with all nests within about a meter of each other, but no colony center or area with brood can be found.
In different parts of its range, in both polygynous and monogynous forms, Crematogaster carinata may form parabiotic associations with other ants. In the La Selva forest canopy it cohabits large ant gardens with Odontomachus panamensis. Because the ant gardens are conspicuous and packed with Crematogaster workers and brood, it can appear that the Crematogaster are a specialized associate with Odontomachus, but closer inspection reveals that the ant gardens are nodes of higher Crematogaster density in a sea of thinly spread Crematogaster nests. It is possible that the ants in Odontomachus nests are genetically and behaviorally differentiated from those in the diffuse polydomous colonies, but I have not found any evidence for this based on external morphology.
In the Pacific lowlands of Costa Rica I have twice seen parabiotic associations between carinata and Dolichoderus species. At Carara I observed a parabiotic association between carinata and Dolichoderus debilis. Several nests were in a cluster of dead branches. Crematogaster workers and brood were distributed in multiple chambers, and one chamber contained a single physogastric queen. The nests of the two species were contiguous and interdigitated, with interconnections among chambers, but they were still largely segregated.
In general the Crematogaster occupied smaller and more peripheral chambers, while the Dolichoderus occupied larger chambers in the center of the branches. In some peripheral chambers I found workers of both species together, but these chambers never contained brood. Any chamber with brood always contained only one species. In Corcovado National Park I observed parabiotic foraging involving carinata and Dolichoderus inermis. Workers of both species were using the same foraging trails, and both species occurred together in clusters of non-foraging workers. Davidson has multiple observations of parabiotic foraging between carinata and D. debilis in Peru (pers. comm.), and many similar observations are reported by Forel (1898) and Wheeler (1921a).
Wherever I have observed carinata it makes use of carton construction to a variable extent. Construction may be as small as a 2cm diameter shelter over a single scale insect. Several times I have seen a 5-10cm wide globular mass of carton extending from the end of a rotten stick or investing epiphyte roots, extending the nest volume. In very humid areas these small carton nests may sprout epiphyte seedlings and in some cases form small ant gardens. Workers form large and more fully developed ant gardens only when in the presence of a larger parabiotic associate.
Workers may be found foraging day or night, and they are generalized omnivores. I have seen them scavenging dead or wounded insects, they recruit to carbohydrate and protein baits, and they are frequent visitors at extrafloral nectaries.
Further evidence for separate species has been found by D. Davidson at her study site at Cocha Cashu Biological Station in Amazonian Peru. In early studies of ant gardens and their ants (Davidson 1988, Seidel et al. 1990, Davidson et al. 1990) I helped identify the Crematogaster and I failed to differentiate levior and carinata. More recent observations on behavior and defensive chemistry suggest discrete sympatric forms, with levior inhabiting ant gardens with Camponotus and carinata found outside those gardens (Davidson pers. comm.). Davidson has discovered that levior has lost its chemical defense and instead relies on the defensive capabilities of C. femoratus. In contrast, carinata has retained chemical defenses. Reflecting the differences in defensive chemistry, levior does not elevate the gaster when disturbed; carinata does. Crematogaster carinata is often found living parabiotically with Dolichoderus debilis, and in this case it is the Dolichoderus that has lost its chemical defense, relying on the Crematogaster. Davidson's observations on carinata in Peru not withstanding, one should not assume that carinata exhibits the same behavior and defensive chemistry throughout the range. It would be worth investigating whether carinata at La Selva Biological Station, which lives in ant gardens with the aggressive species Odontomachus panamensis, exhibits behavior and chemistry more like levior. In contrast, the carinata from Costa Rica's Pacific slope, which is monogynous and parabiotic with non-aggressive Dolichoderus, might be like Peruvian carinata.
Wheeler and Darlington (1930) - A small, inoffensive ant which often colonizes single branches of trees inhabited by Pseudomyrmex mordax and/or Pseudomyrmex triplarinus. It appears to be able to maintain colonies without leaving their home tree.
De Oliveira et al. (2015), studying ant occupancy of Cecropia trees in southwest Bahia, Brazil, found a colony of Crematogaster carinata opportunistically nesting in a Cecropia pachystachya tree. Gillette et al. (2015) in a Chaipas, Mexico field study of twig-nesting ants in coffee plants found C. carinata nesting within one sampled plot of plants at 500m.
The occurrence of ant gardens in this species is discussed by Campbell et al. (2022), Davidson (1988), Dejean et al. (2000), Kleinfeldt (1986), Longino (2003), Marini Filho (1999), Orivel & Leroy (2011), Schmit-Neuerburg & Bluthgen (2007), Weber (1943), Wheeler (1921) and Youngsteadt et al. (2009).
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Crematogaster carinata has been found in parabiotic associations with the following species:
- Dolichoderus bispinosus
- Dolichoderus debilis
- Dolichoderus inermis
- Odontomachus panamensis
- Solenopsis picea
- Temnothorax subditivus
This species is a host for the phorid fly Pseudacteon sp. (a parasitoid) (Quevillon, 2018) (encounter mode primary; direct transmission; transmission outside nest).
Life History Traits
- Queen number: polygynous (Frumhoff & Ward, 1992)
Castes
Worker
                   
| |
| . | |
Queen
Images from AntWeb
 
| |
| Queen (alate/dealate). Specimen code casent0627993. Photographer C. Richart, uploaded by University of Utah. | Owned by INBio. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- carinata. Crematogaster carinata Mayr, 1862: 768 (w.) BRAZIL (Rio de Janeiro).
- Type-material: lectotype worker (by designation of Longino, 2003a: 44), 1 paralectotype worker.
- Type-locality: lectotype Brazil: Rio de Janeiro (Novara Expd.) (sent by R. von Frauenfeld); paralectotype with same data.
- Type-depositories: NMHW (lectotype); MSNG (paralectotype).
- Longino, 2003a: 46 (q.).
- Combination in C. (Orthocrema): Emery, 1922e: 136.
- Status as species: Mayr, 1863: 404; Roger, 1863b: 37; Mayr, 1865: 105 (redescription); Mayr, 1870b: 990 (in key); Dalla Torre, 1893: 80; Forel, 1895b: 131; Emery, 1922e: 136; Wheeler, W.M. 1923c: 4; Borgmeier, 1927c: 93; Menozzi, 1935b: 195; Kempf, 1972a: 86; Bolton, 1995b: 149; Longino, 2003a: 44 (redescription); Branstetter & Sáenz, 2012: 258; Bezděčková, et al. 2015: 116; Wetterer, et al. 2016: 23; Pedraza & Fernández, 2019: 895.
- Senior synonym of parabiotica: Longino, 2003a: 44.
- Distribution; Bolivia, Brazil, Colombia, Costa Rica, Guatemala, Panama, Peru, Trinidad, Venezuela.
- parabiotica. Crematogaster limata r. parabiotica Forel, 1904e: 683 (w.) COLOMBIA, BRAZIL (Pará, Amazonas), COSTA RICA.
- Type-material: lectotype worker (by designation of Longino, 2003a: 44); paralectotype workers (number not stated).
- Type-locality: lectotype Colombia: Magdalena, Orihueca, in parabiosis with Dolichoderus debilis (A. Forel)
- (invalid restriction of type-locality by Kempf, 1972a: 88; no lectotype designated),
- [Note: other original syntype localities: Brazil: Pará (E.A. Göldi), Brazil: Amazonas, Jurua, Jurua Miry, Tillandsia no. 5734 (E. Ule), Costa Rica: (no further data) (H. Pittier).]
- Type-depository: MHNG.
- Forel, 1912f: 217 (q.).
- Combination in C. (Orthocrema): Emery, 1922e: 136.
- Status as species: Wheeler, W.M. 1921b: 99 (in text); Wheeler, W.M. & Darlington, 1930: 109.
- Subspecies of limata: Forel, 1907e: 6; Forel, 1911e: 274; Forel, 1912f: 217; Stitz, 1913: 209; Wheeler, W.M. 1916c: 12; Wheeler, W.M. 1916d: 324; Luederwaldt, 1918: 42; Wheeler, W.M. 1918b: 24; Wheeler, W.M. 1921f: 152; Wheeler, W.M. 1922c: 8; Emery, 1922e: 136; Wheeler, W.M. 1923a: 3; Borgmeier, 1927c: 95; Kutter, 1931a: 61; Santschi, 1939f: 161; Wheeler, W.M. 1942: 197; Kempf, 1972a: 88; Brandão, 1991: 338; Bolton, 1995b: 159.
- Junior synonym of carinata: Longino, 2003a: 44.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Longino (2003) - HL 0.561, 0.528, 0.657; HW 0.604, 0.563, 0.722; HC 0.565, 0.507, 0.664; SL 0.485, 0.512, 0.612; EL 0.130, 0.139, 0.175; A11L 0.215; A11W 0.101; A10L 0.113; A10W 0.089; A09L 0.068; A09W 0.064; A08L 0.051; A08W 0.051; WL 0.615, 0.585, 0.744; SPL 0.077, 0.109, 0.148; PTH 0.148, 0.137, 0.166; PTL 0.207, 0.213, 0.250; PTW 0.144, 0.138, 0.174; PPL 0.169, 0.129, 0.178; PPW 0.162, 0.119, 0.166; CI 108, 107, 110; OI 23, 26, 27; SI 86, 97, 93; PTHI 71, 64, 66; PTWI 70, 65, 70; PPI 96, 92, 93; SPI 13, 19, 20; ACI 0.64.
Color red brown; mandibles, antennal club, and tarsi usually lighter yellow; workers monomorphic in size.
Mandibles smooth and shining; clypeus usually with 5-6 longitudinal carinulae, these may be more abundant, making clypeus uniformly striate, or they may be nearly absent, making clypeus smooth and shining, especially medially; head about as long as wide, subquadrate, with emarginate posterior border; antenna with distinct two-segmented club, or third segment from end somewhat enlarged, blurring distinction between two and three-segmented club; scapes with abundant long erect setae; when scapes laid back from antennal insertions, they slightly surpass margin of vertex; face largely smooth and shining, with variable extent of striated region between antennal insertion and eye, and whorled above antennal insertion; face covered with abundant long flexuous white setae, no appressed pubescence; in face view abundant setae project from lateral and posterior margins.
Promesonotum in profile forming evenly convex surface, varying from flattish to forming peak at juncture of pro and mesonotum; propodeal suture deep in dorsal view but obscured in profile due to lateral carinulae that bridge the suture; posterior mesonotum curves smoothly into horizontal dorsal face of propodeum; propodeal spines short, projecting posteriorly such that they are more or less in same plane as dorsal face of propodeum; dorsal and posterior face of propodeum appear well differentiated in lateral view, the dorsal face confluent with the horizontal spines, the posterior face sloping down to petiolar insertion, but faces less differentiated medially; pronotal dorsum with variably developed longitudinal carinulae, strongest laterally, becoming weaker medially, interspaces smooth and shining; mesonotal dorsum with two strong, subparallel lateral carinae, interspace smooth and shining or with faint longitudinal carinulae; dorsal face of propodeum striatorugose, rugulae extending onto spines, posterior face smooth and shining; side of pronotum smooth and shining; katepisternum and ventral portion of side of propodeum variously punctatorugose; dorsal portion of side of propodeum smooth and shining; mesosomal dorsum with abundant long flexuous white setae, setae on pronotal humeri longest; femora and tibiae with abundant long erect setae.
Petiole in side view subtrapezoidal, varying in length and degree of narrowing anteriorly, weakly punctate to nearly smooth; anteroventral tooth well developed, often forming a right-angle to short acute tooth; dorsal face of petiole smooth and shining, elongate, widest posteriorly, regularly tapering anteriorly, with a long flexuous seta on each posterolateral tubercle and varying number of short setae along posterior border; postpetiole lacking ventral tooth, globular in dorsal view, with abundant erect setae; fourth abdominal tergite smooth and shining, with abundant long flexuous erect white setae, no appressed pubescence.
Queen
Longino (2003) - A normal queen (dorsal face of propodeum drops steeply from postscutellum and much of propodeum appears ventral to scutellum and postscutellum) with general shape, sculpture, and pilosity characters of the worker; size characters as in Figures.
Type Material
Longino (2003) - Syntype workers: Brazil, Rio de Janeiro (Novara) Naturhistorisches Museum Wien, Vienna, Museo Civico di Storia Naturale, Genoa (examined, NMW worker here designated LECTOTYPE).
References
- Adams, R.M.M., Wells, R.L., Yanoviak, S.P., Frost, C.J., Fox, E.G.P. 2020. Interspecific Eavesdropping on Ant Chemical Communication. Frontiers in Ecology and Evolution 8. (doi:10.3389/fevo.2020.00024).
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Campbell, L.C.E., Kiers, E.T., Chomicki, G. 2022. The evolution of plant cultivation by ants. Trends in Plant Science (doi:10.1016/j.tplants.2022.09.005).
- Davidson, D.W. 1988. Ecological studies of neotropical ant gardens. Ecology 69: 1138-1152.
- de Oliveira, G. V., M. M. Correa, I. M. A. Goes, A. F. P. Machado, R. J. de Sa-Neto, and J. H. C. Delabie. 2015. Interactions between Cecropia (Urticaceae) and ants (Hymenoptera: Formicidae) along a longitudinal east-west transect in the Brazilian Northeast. Annales De La Societe Entomologique De France. 51:153-160. doi:10.1080/00379271.2015.1061231
- Dejean, A., Corbara, B., Orivel, J., Snelling, R.R., Delabie, J.H.C., Belin-Depoux, M. 2000. The importance of ant gardens in the pioneer vegetal formations of French Guiana. Sociobiology 35: 425-439.
- Emery, C. 1922c. Hymenoptera. Fam. Formicidae. Subfam. Myrmicinae. [part]. Genera Insectorum 174B: 95-206 (page 136, Combination in C. (Orthocrema))
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Gillette, P. N., K. K. Ennis, G. D. Martinez, and S. M. Philpott. 2015. Changes in Species Richness, Abundance, and Composition of Arboreal Twig-nesting Ants Along an Elevational Gradient in Coffee Landscapes. Biotropica. 47:712-722. doi:10.1111/btp.12263
- Khazan, E., Bujan, J., Scheffers, B.R. 2020. Patterns of ant activity and nesting ecology depend on flooding intensity in a Neotropical floodplain. International Journal of Tropical Insect Science (doi:10.1007/s42690-020-00149-0).
- Kleinfeldt, S.E. 1986. Ant-gardens: mutual exploitation. In: Juniper, B. & Southwood, T.R.E. (Eds.): Insects and the plant surface. Edward Arnold, Oxford, pp. 283-291.
- Longino, J.T. 2003a. The Crematogaster of Costa Rica. Zootaxa 151: 1-150. (page 44, worker, queen described, Senior synonym of parabiotica)
- Marini-Filho, O.J. 1999. Distribution, composition, and dispersal of ant gardens and tending ants in three kinds of central Amazonian habitats. Tropical Zoology 12: 289-296.
- Mayr, G. 1862. Myrmecologische Studien. Verh. K-K. Zool.-Bot. Ges. Wien 12: 649-776 (page 768, worker described)
- Orivel, J., Leroy, C. 2011. The diversity and ecology of ant gardens (Hymenoptera: Formicidae; Spermatophyta: Angiospermae). Myrmecological News 14: 73-85.
- Przybyszewski, K.R., Silva, R.J., Vicente, R.E., Garcia Freitas, J.V., Pereira, M.J.B., Izzo, T.J., Tonon, D.S. 2020. Can baited pitfall traps for sampling dung beetles replace conventional traps for sampling ants? Sociobiology 67, 376-387 (doi:10.13102/sociobiology.v67i3.5201).
- Schmit-Neuerburg, V. & Bluthgen, N. 2007. Ant-garden epiphytes are protected against drought in a venezuelan lowland rainforest. Ecotropica 13: 93-100.
- Weber, N.A. 1943. Parabiosis in neotropical "ant gardens". Ecology 24: 400-404.
- Wheeler, W.M. 1921. A new case of parabiosis and the "ant gardens" of British Guiana. Ecology 2: 89-103.
- Wheeler, W. M. and P. J. Darlington, Jr. 1930. Ant-tree notes from Rio Frio, Colombia. Psyche. 37:107-117.
- Yanoviak, S.P., Frederick, D.N. 2014. Water surface locomotion in tropical canopy ants. Journal of Experimental Biology 217, 2163–2170 (doi:10.1242/jeb.101600).
- Youngsteadt, E., Alvarez Baca, J., Osborne, J. & Schal, C. 2009. Species-specific seed dispersal in an obligate ant-plant mutualism. PLOS ONE 4: e4335.
References based on Global Ant Biodiversity Informatics
- Achury R., and A.V. Suarez. 2017. Richness and composition of ground-dwelling ants in tropical rainforest and surrounding landscapes in the Colombian Inter-Andean valley. Neotropical Entomology https://doi.org/10.1007/s13744-017-0565-4
- Adams B. J., S. A. Schnitzer, and S. P. Yanoviak. 2016. Trees as islands: canopy ant species richness increases with the size of liana-free trees in a Neotropical forest. Ecography doi: 10.1111/ecog.02608
- Adams B. J., S. A. Schnitzer, and S. P. Yanoviak. 2019. Connectivity explains local ant community structure in a Neotropical forest canopy: a large-scale experimental approach. Ecology 100(6): e02673.
- Armbrecht I., E. Jimenez, G. Alvarez, P. Ulloa-Chacon, and H. Armbrecht. 2001. An ant mosaic in the Colombian rain forest of Choco (Hymenoptera: formicidae0. Sociobiology 37(3B): 491-509.
- Astruc C., J. F. Julien, C. Errard, and A. Lenoir. 2004. Phylogeny of ants based on morphology and DNA sequence data. Molecular Phylogenetics and Evolution 31: 880-893.
- Bezdeckova K., P. Bedecka, and I. Machar. 2015. A checklist of the ants (Hymenoptera: Formicidae) of Peru. Zootaxa 4020 (1): 101–133.
- Brandao, C.R.F. 1991. Adendos ao catalogo abreviado das formigas da regiao neotropical (Hymenoptera: Formicidae). Rev. Bras. Entomol. 35: 319-412.
- Castano-Meneses G., R. De Jesus Santos, J. R. Mala Dos Santos, J. H. C. Delabie, L. L. Lopes, and C. F. Mariano. 2019. Invertebrates associated to Ponerine ants nests in two cocoa farming systems in the southeast of the state of Bahia, Brazil. Tropical Ecology 60: 52–61.
- Chacon de Ulloa P., A. M. Osorio-Garica, R. Achury, and C. Bermudez-Rivas. 2012. Hormigas (Hymenoptera: Formicidae) del Bosque seco tropical (Bs-T) de la cuenca alta del rio Cauca, Colombia. Biota Colombiana 13(2): 165-181.
- Cover S. P., J. E. Tobin, and E. O. Wilson. 1990. The ant community of a tropical lowland rainforest site in Peruvian Amazonia. Pp. 699-700 in: Veeresh, G. K.; Mallik, B.; Viraktamath, C. A. (eds.) 1990. Social insects and the environment. Proceedings of the 11th International Congress of IUSSI, 1990. New Delhi: Oxford & IBH Publishing Co., xxxi + 765 pp.
- Dattilo W. et al. 2019. MEXICO ANTS: incidence and abundance along the Nearctic-Neotropical interface. Ecology https://doi.org/10.1002/ecy.2944
- Davidson, D.W., J.A. Arias and J. Mann. 2006. An experimental study of bamboo ants in western Amazonia. Insectes Sociaux 53:108-114
- Davidson, D.W., J.A. Arias and J. Mann. 2006. An experimental study of bamboo ants in western Amazonia. Insectes Sociaux 53:108114.
- De la Mora, A., J. A. Garcia-Ballinas, and S. M. Philpott. 2015. Local, landscape, and diversity drivers of predation services provided by ants in a coffee landscape in Chiapas, Mexico. Agriculture, Ecosystems & Environment 201: 83-91.
- Fernandes I., and J. de Souza. 2018. Dataset of long-term monitoring of ground-dwelling ants (Hymenoptera: Formicidae) in the influence areas of a hydroelectric power plant on the Madeira River in the Amazon Basin. Biodiversity Data Journal 6: e24375.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Fichaux M., B. Bechade, J. Donald, A. Weyna, J. H. C. Delabie, J. Murienne, C. Baraloto, and J. Orivel. 2019. Habitats shape taxonomic and functional composition of Neotropical ant assemblages. Oecologia 189(2): 501-513.
- Forel A. 1904. In und mit Pflanzen lebende Ameisen aus dem Amazonas-Gebiet und aus Peru, gesammelt von Herrn E. Ule. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 20: 677-707.
- Forel A. 1912. Formicides néotropiques. Part III. 3me sous-famille Myrmicinae (suite). Genres Cremastogaster et Pheidole. Mémoires de la Société Entomologique de Belgique. 19: 211-237.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Gibernau M., J. Orivel, J. H. C. Delabie, D. Barabe, and A. Dejean. 2007. An asymmetrical relationship between an arboreal ponerine ant and a trash-basket epiphyte (Araceae). Biological Journal of the Linnean Society 91: 341-346.
- Gomez V. E. S., and G. Z. González. 2007. Catalogo de Las Hormigas Presentes en El Museo de Historia Natural de la Universidad del Cauca. Popayán : 1-58.
- Groc S., J. H. C. Delabie, F. Fernandez, M. Leponce, J. Orivel, R. Silvestre, Heraldo L. Vasconcelos, and A. Dejean. 2013. Leaf-litter ant communities (Hymenoptera: Formicidae) in a pristine Guianese rainforest: stable functional structure versus high species turnover. Myrmecological News 19: 43-51.
- INBio Collection (via Gbif)
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Larsen, A., and S. M. Philpott. 2010. Twig-nesting ants: the hidden predators of the coffee berry borer in Chiapas, Mexico. Biotropica 42: 342-347.
- Livingston G. F., S. M. Philpott, and A. de la Mora Rodriguez. 2012. Do species sorting and mass effects drive assembly in tropical agroecological landscape mosaics? Biotropica 45(1): 10-17.
- Longino J. T. 2013. Ants of Nicargua. Consulted on 18 Jan 2013. https://sites.google.com/site/longinollama/reports/ants-of-nicaragua
- Longino J. T. L., and M. G. Branstetter. 2018. The truncated bell: an enigmatic but pervasive elevational diversity pattern in Middle American ants. Ecography 41: 1-12.
- Longino J. et al. ADMAC project. Accessed on March 24th 2017 at https://sites.google.com/site/admacsite/
- Longino, J.T. 2003. The Crematogaster (Hymenoptera, Formicidae, Myrmicinae) of Costa Rica. Zootaxa 151:1-150
- Luederwaldt H. 1918. Notas myrmecologicas. Rev. Mus. Paul. 10: 29-64.
- Menozzi C. 1935. Spedizione del Prof. Nello Beccari nella Guiana Britannica. Hymenoptera-Formicidae. Redia. 21: 189-203.
- Miranda P. N., F. B. Baccaro, E. F. Morato, M. A. Oliveira. J. H. C. Delabie. 2017. Limited effects of low-intensity forest management on ant assemblages in southwestern Amazonian forests. Biodivers. Conserv. DOI 10.1007/s10531-017-1368-y
- Philpott S. M., I. Perfecto, and J. Vandermeer. 2006. Effects of management intensity and season on arboreal ant diversity and abundance in coffee agroecosystems. 15: 139-155.
- Philpott, S. M. 2006. Ant patchiness: a spatially quantitative test in coffee agroecosystems. Naturwissenschaften 93: 386-392.
- Ryder Wilkie K.T., A. L. Mertl, and J. F. A. Traniello. 2010. Species Diversity and Distribution Patterns of the Ants of Amazonian Ecuador. PLoS ONE 5(10): e13146.doi:10.1371/journal.pone.0013146
- Sandoval V. E., and G. Zambrano. 2007. Catálogo de las hormigas presentes en el Museo de Historia Natural de la Universidad del Cauca. Taller Editorial de la Universidad del Cauca, Popayán. 60 pp.
- Wheeler W. M. 1916. Ants collected in British Guiana by the expedition of the American Museum of Natural History during 1911. Bulletin of the American Museum of Natural History 35: 1-14.
- Wheeler W. M. 1918. Ants collected in British Guiana by Mr. C. William Beebe. Journal of the New York Entomological Society 26: 23-28.
- Wheeler W. M. 1922. The ants of Trinidad. American Museum Novitates 45: 1-16.
- Wheeler W. M. 1942. Studies of Neotropical ant-plants and their ants. Bulletin of the Museum of Comparative Zoology 90: 1-262.
- Wheeler, William Morton. 1923. Report on the Ants. The University of Iowa Studies in Natural History. 10(3):3-9.
- Wilson, E.O. 1987. The Arboreal Ant Fauna of Peruvian Amazon Forests: A First Assessment. Biotropica 19(3):245-251.
- Pages using DynamicPageList3 parser function
- Polygynous
- Xenobiotic
- Ant garden
- Tropical
- South subtropical
- South temperate
- Ant Associate
- Host of Dolichoderus bispinosus
- Host of Dolichoderus debilis
- Host of Dolichoderus inermis
- Host of Odontomachus panamensis
- Host of Solenopsis picea
- Host of Temnothorax subditivus
- Phorid fly Associate
- Host of Pseudacteon sp.
- Species
- Extant species
- Formicidae
- Myrmicinae
- Crematogastrini
- Crematogaster
- Crematogaster carinata
- Myrmicinae species
- Crematogastrini species
- Crematogaster species
- Ssr

