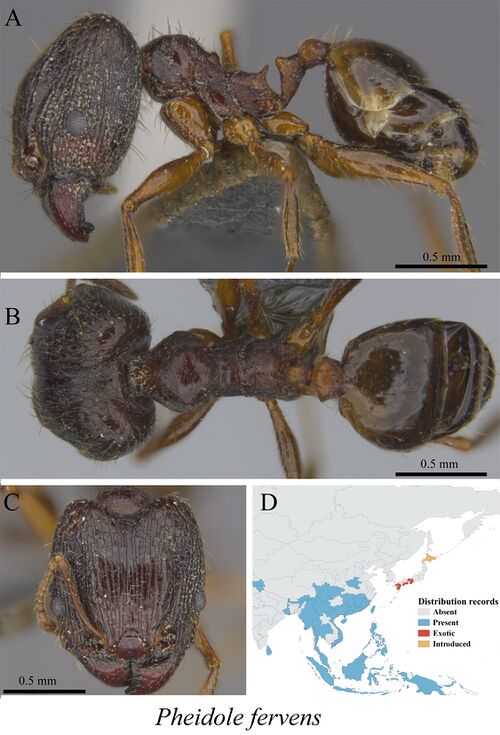
Pheidole fervens
| Pheidole fervens | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Pheidole |
| Species group: | fervens |
| Species: | P. fervens |
| Binomial name | |
| Pheidole fervens Smith, F., 1858 | |
| Subspecies | |
| |
| Synonyms | |
| |
| Common Name | |
|---|---|
| Minami-oozu-ari | |
| Language: | Japanese |
Fischer & Fisher (2013): Pheidole fervens, described from Singapore, is a widespread invasive species and could be native to the Oriental or the Oceanic region (Eguchi 2004, Wilson & Taylor 1967). It usually nests in soil or under stones and usually prefers disturbed habitats (Eguchi 2004). In Japan it is found nesting under stones in open land grading to forest margins (Ogata, 1981). On Fiji it was collected in elevations between 1–800 m and tended to inhabit several different habitats from forest edge and mangrove forest to disturbed and undisturbed rainforest. On Mauritius, which seems to be its western distribution limit, this ant was found in the leaf litter of lowland rainforest (200 m elevation). As with most other introduced ants, such as Pheidole indica, it is unclear what effect, if any, P. fervens might have on the rest of the local ant fauna. Considering that the ecosystems of the Mauritius islands have been severely altered and disturbed since the arrival of human settlers several hundred years ago, and native species must also contend with the invasions of introduced organisms (Fisher 2005, Ward 1990), parts of the original ant fauna might have been marginalized or driven to extinction long ago. We speculate that the scarcity of P. fervens specimens in Mauritius ant collections and its presence in the rainforest means that this ant has not yet become an invasive and dominant aggressor toward other ant species. Nevertheless, efforts to further investigate the presence and activity of introduced Pheidole species are worthwhile, especially in the few remaining undisturbed habitats.
Pashaei Rad et al. (2018) found this species in Iran on the ground in moist to moderate rainfall areas.
| At a Glance | • Invasive |
Identification
Fischer and Fisher (2013) - Large species (WL major 1.06–1.17, WL minor 0.82–0.91), minor workers with relatively long scapes and legs (SI 133–154, FI 142–158) and major workers with long scapes and relatively long legs (SI 61–71, FI 84–91), both with promesonotal processes well developed and postpetiolar ventral process inconspicuous or very shallow. Major with frontal carinae reaching at least 4/5 of the distance to the posterior head margin, face longitudinally rugose, scrobe area and interspaces at sides of head punctate to weakly punctate, head in full-face view with coarse suberect hairs, eyes moderately large (EI 13–15), in profile metanotal groove vestigial to absent, propodeum sloped down toward spines, postpetiole in dorsal view trapezoidal and almost twice as wide as petiole (PpWI 172-195). Minor head oval and rounded posteriorly, occipital carina visible in full-face view but not well developed, face mostly smooth, often with fine, long rugulae at the sides, sometimes together with superficial punctures. Eyes moderately large (EI 21–25), dorsal promesonotum smooth, promesonotal process developed, subangulate in profile, metanotal groove well developed, impressed, propodeum relatively high and sloped toward spines.
Southwest Indian Ocean
Fischer and Fisher (2013) - The workers of P. fervens can be easily confused with those of Pheidole indica. Morphologically the minor workers are best separated by coarse, mostly suberect scape pilosity, impressed metanotal groove, relatively smaller eyes and shorter spines (EI 21–25, PSLI 10–13) in P. fervens versus decumbent to subdecumbent scape pilosity with longer suberect hairs along outer edge, inconspicuous metanotal groove in profile, and larger eyes and longer spines (EI 25–30, PSLI 14–19) in P. indica. The major workers of P. fervens are recognizable by having longer scapes (SI 61–71 versus SI 49–62) and a narrower postpetiole in dorsal view than P. indica (DPpI 133–150 and PpWI 172–195 versus DPpI 146–235 and PpWI 190–247). In P. fervens, the pilosity in the face and on the metatibia is coarser and at least partly suberect, and the punctures on the sides of the face are stronger than the thinner and more decumbent to subdecumbent pilosity and superficial punctures on the sides of the face in P. indica.
Indo-China
Eguchi (2008) - This medium-sized species with general habitus is similar to Pheidole binghamii, Pheidole elongicephala, Pheidole indica, Pheidole ochracea and Pheidole plagiaria among Indo-Chinese species. P. fervens is well separated from P. indica which has the following characteristics: eye relatively large (maximal diameter of eye much longer than antennal segment X in the major, and as long as or a little longer than X in the minor); in the major propodeal spine is relatively broadly based (see Eguchi 2004).
Posterior margin of head in full-face view is more deeply and narrowly concave in the major of P. binghamii, P. elongicephala and P. ochracea than in that of P. fervens, and sculpture on dorsum of vertexal lobe is usually stronger in the major of the former three.
The major of P. fervens is separated from that of Pheidole plagiaria which has the following characteristics: posterior margin of head in full-face view deeply concave; the rugulae running almost transversely along posterior margin of vertexal lobe.
Japan
Habitat preference, body size and coloration are similar to Pheidole indica, Pheidole nodus and Pheidole megacephala. In fact, these species are sometimes closely sympatric in the Ryukyus. P. fervens can be distinguished by its lighter body color, smaller eyes, the relatively fine reticulation on the heads of its soldiers, the shape of the propodeal spines and the proportions of its postpetiole (Japanese Ant Image Database).
Keys including this Species
- Key to the Pheidole of North Vietnam
- Key to Micronesian Ants
- Key to Pheidole of the islands of the Southwest Indian Ocean
- Key to Pheidole majors and minors of Borneo
- Key to Pheidole majors of Borneo
- Key to Pheidole minors of Borneo
Distribution
Known from N. Vietnam, Manchurian subregion (southern part only), Oriental region, Austro-Malayan subregion, W. Pacific and West coast of N. America. In the Indo-Malayan subregion this species is one of the prevailing Pheidole species in semiurban and rural areas. (Eguchi 2008)
Latitudinal Distribution Pattern
Latitudinal Range: 32.812778° to -3.7°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Australasian Region: New Caledonia.
Indo-Australian Region: Borneo, Cook Islands, Fiji, French Polynesia, Guam, Indonesia (type locality), Kiribati, Krakatau Islands, Malaysia, Marshall Islands, Micronesia (Federated States of), Northern Mariana Islands, Palau, Philippines, Pitcairn, Samoa (type locality), Singapore (type locality), Tokelau, Tonga, Vanuatu.
Malagasy Region: Mauritius.
Nearctic Region: United States.
Oriental Region: India (type locality), Sri Lanka, Taiwan (type locality), Thailand, Vietnam.
Palaearctic Region: China (type locality), Iran, Japan (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
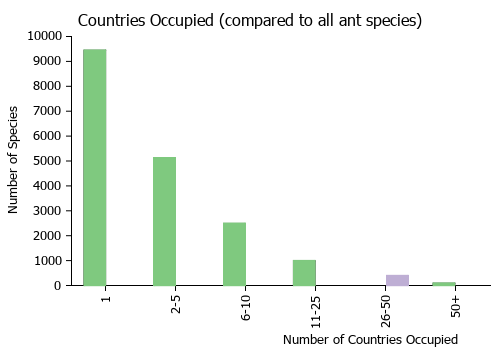
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a mutualist for the aphid Aphis gossypii (a trophobiont) (Idechiil et al., 2007; Saddiqui et al., 2019).
Castes
Worker
Minor
Images from AntWeb
   
| |
| Worker. Specimen code casent0005780. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by UCDC, Davis, CA, USA. |
   
| |
| . | |
  
| |
| Worker. Specimen code casent0008639. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by UCDC, Davis, CA, USA. |
Major
| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- fervens. Pheidole fervens Smith, F. 1858b: 176 (s.) SINGAPORE. Ogata, 1982: 197 (m.). Senior synonym of cavannae, javana, nigriscapa and material of the unavailable name tahitiana referred here: Wilson & Taylor, 1967: 45; senior synonym of desucta: Eguchi, 2001b: 53; of amia, azumai, dharmsalana, dolenda, pungens, soror: Eguchi, 2004: 197. Current subspecies: nominal plus pectinata.
- pungens. Solenopsis pungens Smith, F. 1861b: 48 (s.w.) INDONESIA (Sulawesi).
- Combination in Pheidologeton: Emery, 1924d: 213; Donisthorpe, 1932c: 469; Chapman & Capco, 1951: 160;
- Combination in Carebara: Bolton, 1995b: 333 (error); Fischer, et al. 2014: 72 (error);
- Combination in Pheidole: Bolton, 1995b: 328.
- Status as species: Roger, 1863b: 32; Mayr, 1863: 454; Smith, F. 1871a: 333; Dalla Torre, 1893: 77; Emery, 1924d: 213; Donisthorpe, 1932c: 469; Chapman & Capco, 1951: 160; Bolton, 1995b: 328.
- Junior synonym of fervens: Eguchi, 2004: 198; Eguchi, 2008: 24; Fischer & Fisher, 2013: 322; Sarnat, et al. 2015: 38.
- javana. Pheidole javana Mayr, 1867a: 98 (s.w.) INDONESIA (Java). Viehmeyer, 1916a: 121 (q.m.). Junior synonym of fervens: Wilson & Taylor, 1967: 45.
- cavannae. Pheidole cavannae Emery, 1887b: 464 (footnote) (s.) NEW CALEDONIA. Subspecies of oceanica: Emery, 1914f: 401. Junior synonym of fervens: Wilson & Taylor, 1967: 45.
- dharmsalana. Pheidole javana var. dharmsalana Forel, 1902c: 184 (s.), 198 (w.) INDIA. [Also described as new by Forel, 1902f: 546.] Subspecies of fervens: Bolton, 1995b: 320. Junior synonym of fervens: Eguchi, 2004: 198.
- amia. Pheidole amia Forel, 1912a: 60 (s.w.) TAIWAN. Junior synonym of fervens: Eguchi, 2004: 197.
- dolenda. Pheidole javana var. dolenda Forel, 1912a: 60 (s.w.) TAIWAN. Subspecies of fervens: Bolton, 1995b: 320. Junior synonym of fervens: Eguchi, 2004: 198.
- nigriscapa. Pheidole (Pheidole) oceanica var. nigriscapa Santschi, 1928a: 48, fig. 3 (s.w.) SAMOA. Junior synonym of fervens: Wilson & Taylor, 1967: 45.
- desucta. Pheidole javana var. desucta Wheeler, W.M. 1929f: 2 (s.w.q.) CHINA. Subspecies of fervens: Bolton, 1995b: 320. Junior synonym of fervens: Eguchi, 2001b: 53.
- soror. Pheidole javana st. soror Santschi, 1937h: 369, fig. 6 (s.w.) TAIWAN. Subspecies of fervens: Bolton, 1995b: 330. Junior synonym of fervens: Eguchi, 2004: 198.
- azumai. Pheidole nodus st. azumai Santschi, 1941: 274, fig. 1 (s.w.) JAPAN. Junior synonym of fervens: Eguchi, 2004: 198.
Type Material
- Pheidole fervens: Lectotype (designated by Fischer & Fisher, 2013: 322), major worker, Singapore, The Natural History Museum.
- Pheidole fervens: Paralectotype (designated by Fischer & Fisher, 2013: 322), 1 minor worker, Singapore, The Natural History Museum.
- Solenopsis pungens: Lectotype (designated by Eguchi, 2004), major worker, Sulawesi, Indonesia, Oxford University Museum of Natural History.
- Pheidole javana: Lectotype (designated by Eguchi, 2004), major worker, Java, Indonesia.
- Pheidole javana dharmsalana: Lectotype (designated by Eguchi, 2004), major worker, India.
- Pheidole amia: Lectotype (designated by Eguchi, 2004), major worker, Taiwan.
- Pheidole javana dolenda: Lectotype (designated by Eguchi, 2004), major worker, Taiwan.
- Pheidole oceanica nigriscapa: Syntype, 1 major worker, 7 minor workers, Samoa.
- Pheidole javana desucta: Lectotype (designated by Eguchi, 2001), major worker, China.
- Pheidole javana soror: Lectotype (designated by Eguchi, 2004), major worker, Taiwan.
- Pheidole nodus azumai: Lectotype (designated by Eguchi, 2004), major worker, Japan.
The following notes on F. Smith type specimens have been provided by Barry Bolton (details):
Solenopsis pungens
Two syntype major workers and one syntype minor worker (on separate cards) in Oxford University Museum of Natural History. Each major worker labelled “Menado” and with a Smith det. label “Solenopsis pungens Smith.” The minor worker with “Men. 18” and an attached note explaining that Pheidole pungens was placed in Solenopsis by an oversight. Each of the three cards has the letter “c” in the lower left corner.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Eguchi (2008) - Major (n=5). — HL 1.31–1.56 mm; HW 1.22–1.44 mm; CI 92–97; SL 0.84–0.95 mm; SI 66–70; FL 1.12–1.27 mm; FI 88–94. Minor (n=5). — HL 0.65–0.73 mm; HW 0.54–0.60 mm; CI 81–84; SL 0.77–0.87 mm; SI 138–153; FL 0.81–0.93 mm; FI 149–163.
Head in lateral view not or hardly impressed on vertex; posterior concavity of head in full-face view usually shallow; frons and anterior part of vertex rugose longitudinally; posterior part of vertex and dorsal face of vertexal lobe rugoso-reticulate, with interspaces weakly punctured; frontal carina conspicuous; antennal scrobe inconspicuous or shallowly impressed; clypeus without a median longitudinal carina; median and submedian processes of hypostoma absent or present but inconspicuous; lateral processes conspicuous; antenna with a 3-segmented club; maximal diameter of eye almost as long as or shorter than antennal segment X. Promesonotal dome in dorsal view largely smooth, or sparsely sculptured with weak transverse rugulae, in lateral view with a low to conspicuous mound on its posterior slope; humerus not produced; the dome much narrower at the humeri than at the bottom; mesopleuron, metapleuron and lateral face of propodeum well punctured, sometimes overlain with weak rugulae; propodeal spine narrowly based, usually slightly curved apically. Petiole longer than postpetiole (excluding helcium); postpetiole not massive. First gastral tergite smooth and shining entirely, or sometimes shagreened around its articulation with postpetiole.
Minor — Frons and vertex smooth and shining, or rarely shagreened or very weakly rugoso-punctate; area between antennal insertion and eye weakly rugoso-punctate; preoccipital carina conspicuous dorsally and laterally; median part of clypeus smooth, without a median longitudinal carina; antenna with a 3-segmented club; scape extending far beyond posterolateral margin of head; maximal diameter of eye much shorter than antennal segment X. Promesonotal dome largely smooth and shining, but sometimes weakly rugoso-punctate dorsolaterally, in lateral view with a low or inconspicuous mound on its gentle posterior slope; humerus of the dome in dorsal-oblique view not produced; mesopleuron, metapleuron and lateral face of propodeum well punctured; propodeal spine elongate-triangular, directing upward. Petiole (a little) longer than postpetiole (excluding helcium); postpetiole not massive.
Fischer and Fisher (2013) - Major (n=5): HW 1.13–1.31 (1.25), HL 1.13–1.38 (1.28), SL 0.80– 0.86 (0.83), MDL 0.61–0.71 (0.67), EL 0.16–0.17 (0.17), WL 1.06–1.17 (1.14), PNH 0.41–0.49 (0.45), PNW 0.54–0.59 (0.56), MNH 0.72–0.79 (0.77), PDH 0.34–0.39 (0.36), PTL 0.36–0.40 (0.38), PPL 0.23–0.26 (0.24), PTH 0.22–0.26 (0.24), PPH 0.16–0.22 (0.20), PTW 0.17–0.20 (0.19), PPW 0.31–0.39 (0.34), PSL 0.13–0.17 (0.15), MFL 1.03–1.13 (1.08), MTL 0.77–0.84 (0.82), CI 95–100 (98), SI 61–71 (67), MDI 50–56 (53), EI 13–15 (13), FI 84–91 (87), PSLI 10–14 (12), LPpI 110–144 (120), DPpI 133–150 (143), PpWI 172–195 (183), PpLI 60– 68 (64), PpHI 70–95 (86).
Head almost as wide as long (CI 95–100), sides convex in full-face view. Mandibles smooth, moderately long (MDI 50–56), clypeus smooth, median carina absent and two weak lateral carinae present. Frontal carinae well developed and reaching at least 4/5 of the distance to posterior head margin, antennal scrobe present and punctate. Face longitudinally rugose, interspaces on frons superficially punctate, sides of head and posterolateral lobes with additional cross-meshes between rugae or weakly reticulate, interspaces punctate or weakly punctate. Scapes long (SI 61–71) with decumbent to subdecumbent pilosity and few longer, erect hairs on outer edge. Eyes well developed, moderately large (EI 13–15). Submedian hypostomal teeth and median process small to inconspicuous. Promesonotum high-domed, convex, posteriorly subangulate, in profile with prominent angulate promesonotal process and transverse groove. Dorsum of pronotum mostly smooth with some weak to superficial transverse rugulae, lateropronotum smooth except for weak to superficial curved rugulae, rest of mesosoma punctate, dorsally more weakly so, metanotal groove in profile not impressed or very shallow, with cross-ribs often reduced, dorsum of propodeum in profile usually weakly sloped toward spines and about as long as posterior declivity. Propodeal spines short-spiniform, acute, sometimes very weakly curved posteriorly, slightly shorter than distance between their bases (PSLI 10–14). Metatibia moderately long (FI 84–91), pilosity mostly subdecumbent, along outer edge also with longer suberect hairs. Postpetiole on average 1.4 times wider than long (DPpI 133–150) and 1.8 times wider than petiole (PpWI 172–195), postpetiole shape in dorsal view trapezoidal to hexagonal, sides angulate, ventral process absent or inconspicuous. Dorsum of petiole weakly to superficially punctate, of postpetiole smooth, remainder of both waist segments punctate. Gaster smooth. Standing hairs relatively coarse and abundant, of medium length to moderately long
Minor (n=5): HW 0.52–0.61 (0.58), HL 0.66–0.71 (0.68), SL 0.80– 0.84 (0.81), MDL 0.40–0.44 (0.42), EL 0.13, WL 0.82–0.91 (0.86), PNH 0.30–0.31 (0.30), PNW 0.35–0.40 (0.37), MNH 0.57–0.59 (0.58), PDH 0.26–0.28 (0.27), PTL 0.22–0.30 (0.24), PPL 0.15–0.18 (0.16), PTH 0.14–0.18 (0.16), PPH 0.14–0.16 (0.15), PTW 0.11–0.13 (0.12), PPW 0.19–0.21 (0.19), PSL 0.06–0.08 (0.07), MFL 0.82– 0.90 (0.85), MTL 0.64–0.69 (0.66), CI 79–88 (85), SI 133–154 (140), MDI 70–77 (73), EI 21–25 (22), FI 142–158 (147), PSLI 10–13 (12), LPpI 50–53 (52), DPpI 117–133 (122), PpWI 161–173 (166), PpLI 61–70 (65), PpHI 89– 100 (96).
Head shape roundly oval, longer than wide (CI 79–88), sides convex, posterior head margin relatively wide and weakly rounded, occipital carina visible in full-face view, narrow. Mandibles moderately long (MDI 70–77), longitudinally rugulose. Clypeus smooth, sometimes with short lateral carinae present. Face mostly smooth to superficially punctate, several strong oblique malar carinae present, reaching posterior eye level, sometimes with weak punctures in between, sculpture fading posterior of eyes. Scapes relatively long, about 1.2 times longer than head (SI 133–154), when laid back surpassing posterior head margin by about the length of eleventh funicular segment, with coarse, mostly suberect pilosity. Pronotum in profile flatly convex and subangulate, posterior promesonotal process well developed, bluntly subangulate and prominently produced, metanotal groove well developed and impressed, with weak to inconspicuous cross-ribs, propodeum slightly longer than high, in profile declining smoothly or convexly toward spines. Propodeal spines very short to short-triangular and acute, much shorter than distance between their bases (PSLI 14–19). Promesonotum usually smooth to mostly smooth, remainder of mesosoma punctate to weakly punctate. Metafemur relatively long (FI 142–158), metatibia mostly with subdecumbent to suberect pilosity. Postpetiole usually slightly longer than high (LPpI 100–113), on average 1.7 times wider (PpWI 161–173) and significantly shorter than petiole (PpLI 61–70), with ventral process inconspicuous or very small. Dorsum of petiolar node and postpetiole mostly smooth, remainder weakly to superficially punctate. Gaster smooth and shiny. Standing hairs moderately long, abundant, and blunt, with shorter subdecumbent to suberect pilosity. Color light to darker reddish brown, head usually darker.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- n = 19, 2n = 38 (Japan) (Imai, 1969) (as Pheidole nodus).
- n = 17 (Crozier, 1975) (as Pheidole nodus).
- n = 18 (Crozier, 1975) (as Pheidole nodus).
- n = 19 (Crozier, 1975) (as Pheidole nodus).
- n = 20 (Crozier, 1975) (as Pheidole nodus).
References
- Brassard, F., Leong, C.-M., Chan, H.-H., Guénard, B. 2021. High diversity in urban areas: How comprehensive sampling reveals high ant species richness within one of the most urbanized regions of the world. Diversity 13, 358 (doi:10.3390/d13080358).
- de Bekker, C., Will, I., Das, B., Adams, R.M.M. 2018. The ants (Hymenoptera: Formicidae) and their parasites: effects of parasitic manipulations and host responses on ant behavioral ecology. Myrmecological News 28: 1-24 (doi:10.25849/myrmecol.news_028:001).
- Eguchi, K. 2001a. A revision of the Bornean species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Tropics Monogr. Ser. 2: 1-154. (page 53, Senior synonym of desucta)
- Eguchi, K. 2004. Taxonomic revision of two wide-ranging Asian ants, Pheidole fervens and P. indica (Insecta: Hymenoptera, Formicidae), and related species. Ann. Naturhist. Mus. Wien B. Bot. Zool. 105(B): 189-209 (page 197, Senior synonym of amia, javana var. dolenda, javana var. soror, nodus st. azumai, pungens)
- Eguchi, K. 2008. A revision of Northern Vietnamese species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Zootaxa 1902:1-118.
- Fischer, G. & Fisher, B.L. 2013. A revision of Pheidole Westwood (Hymenoptera: Formicidae) in the islands of the Southwest Indian Ocean and designation of a neotype for the invasive Pheidole megacephala. Zootaxa 3683, 301–356.
- Fischer, G., Friedman, N.R., Huang, J.-P., Narula, N., Knowles, L.L., Fisher, B.L., Mikheyev, A.S., Economo, E.P. 2020. Socially parasitic ants evolve a mosaic of host-matching and parasitic morphological traits. Current Biology 30, 3639–3646.e4 (doi:10.1016/j.cub.2020.06.078).
- General, D.E.M. 2021. A preliminary checklist of the ants (Hymenoptera: Formicidae) of the Mt. Pantaron Range, Bukidnon Province, Mindanao Island, Philippines. Halteres, 12:4-14 (doi:10.5281/ZENODO.5371745).
- General, D.E.M., Buenavente, P.A.C., Rodriguez, L.J.V. 2020. A preliminary survey of nocturnal ants, with novel modifications for collecting nocturnal arboreal ants. Halteres 11: 1-12 (doi:10.5281/ZENODO.3707151).
- Hasin, S., Tasen, W. 2020. Ant community composition in urban areas of Bangkok, Thailand. Agriculture and Natural Resources 54: 507-514 (doi:10.34044/j.anres.2020.54.5.07).
- Hoffmann, B., Eldridge, J., Marston, C. 2023. The first eradication of an exotic ant species from the entirety of Australia: Pheidole fervens. Management of Biological Invasions, 14(4), 619–624 (doi:10.3391/mbi.2023.14.4.03).
- Imai, H.T., Kihara, A., Kondoh, M., Kubota, M., Kuribayashi, S., Ogata, K., Onoyama, K., Taylor, R.W., Terayama, M., Yoshimura, M., Ugawa, Y. 2003. Ants of Japan. 224 pp, Gakken, Japan.
- Katayama, M., Tsuji, K. 2010. Habitat differences and occurrence of native and exotic ants on Okinawa Island. Entomological Science 13, 425–429 (doi:10.1111/j.1479-8298.2010.00400.x).
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Lee, C.-C., Weng, Y.-M., Lai, L.-C., Suarez, A.V., Wu, W.-J., Lin, C.-C., Yang, C.-C.S. 2020. Analysis of recent interception records reveals frequent transport of arboreal ants and potential predictors for ant invasion in Taiwan. Insects 11, 356 (doi:10.3390/INSECTS11060356).
- Liu, C., Fischer, G., Hita Garcia, F., Yamane, S., Liu, Q., Peng, Y.Q., Economo, E.P., Guénard, B., Pierce, N.E. 2020. Ants of the Hengduan Mountains: a new altitudinal survey and updated checklist for Yunnan Province highlight an understudied insect biodiversity hotspot. ZooKeys 978, 1–171 (doi:10.3897/zookeys.978.55767).
- Ogata, K. 1982. Taxonomic study of the ant genus Pheidole Westwood of Japan, with a description of a new species (Hymenoptera, Formicidae). Kontyû 50: 189-197 (page 197, male described)
- Pashaei Rad, S., Taylor, B., Torabi, R., Aram, E., Abolfathi, G., Afshari, R., Borjali, F., Ghatei, M., Hediary, F., Jazini, F., Heidary Kiah, V., Mahmoudi, Z., Safariyan, F., Seiri, M. 2018. Further records of ants (Hymenoptera: Formicidae) from Iran. Zoology in the Middle East 64, 145-159 (doi:10.1080/09397140.2018.1442301).
- Salata, S., Fisher, B.L. 2021. Taxonomic revision of Madagascan species of the Pheidole fervens species-group (Hymenoptera, Formicidae). PLOS ONE 16, e0244195 (doi:10.1371/journal.pone.0244195).
- Sarnat, E. M., G. Fischer, B. Guenard, and E. P. Economo. 2015. Introduced Pheidole of the world: taxonomy, biology and distribution. Zookeys. 1-109. doi:10.3897/zookeys.543.6050
- Siddiqui, J. A., Li, J., Zou, X., Bodlah, I., Huang, X. 2019. Meta-analysis of the global diversity and spatial patterns of aphid-ant mutualistic relationships. Applied Ecology and Environmental Research 17: 5471-5524 (doi:10.15666/aeer/1703_54715524).
- Smith, F. 1858b. Catalogue of hymenopterous insects in the collection of the British Museum. Part VI. Formicidae. London: British Museum, 216 pp. (page 176, soldier described)
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Wilson, E. O.; Taylor, R. W. 1967b. The ants of Polynesia (Hymenoptera: Formicidae). Pac. Insects Monogr. 14: 1-109 (page 45, senior synonym of cavannae, javana, nigriscapa, and material of the unavailable name tahitiana referred here.)
- Yamane, S., Hosoishi, S. 2020. Identification of the Japanese species of the ant genus Pheidole based on queen characters. Japanese Journal of Entomology 23 (2):37-53.
- Zhong, Y. 2021. Taxonomy of the ant genus Pheidole Westwood (Hymenoptera: Formicidae) from Fujian, China with description of a new species. Entomotaxonomia 43(4): 1-5 (doi:10.11680/entomotax.2021035).
References based on Global Ant Biodiversity Informatics
- Abe T., S. Yamane, and K. Onoyama. Ants collected on the Krakatau Islands 100 years after the great eruptions. Biogeography 14: 65-75.
- André E. 1892. Voyage de M. Chaper à Bornéo. Catalogue des fourmis et description des espèces nouvelles. Mém. Soc. Zool. Fr. 5: 46-55.
- Azuma, S. and M. Kinjo. 1987. Family Formicidae, In Checklist of the insects of Okinawa. The Biological Society of Okinawa, Nishihara. Pages 310-312.
- Bharti H., Y. P. Sharma, M. Bharti, and M. Pfeiffer. 2013. Ant species richness, endemicity and functional groups, along an elevational gradient in the Himalayas. Asian Myrmecology 5: 79-101.
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Chen Y. C., L. Kafle, and C. J. Shih. 2011. Interspecific Competition between Solenopsis invicta and Two Native Ant Species, Pheidole fervens and Monomorium chinense. Journal of Economic Entomology 104(2): 614-621.
- Eguchi K. 2001. A revision of the Bornean species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Tropics Monograph Series 2: 1-154.
- Eguchi K. 2004. Taxonomic revision of two wide-ranging Asian ants, Pheidole fervens and P. indica (Insecta: Hymenoptera, Formicidae), and related species. Annalen des Naturhistorischen Museums in Wien. B, Botanik, Zoologie 105: 189-209
- Eguchi K. 2008. A revision of Northern Vietnamese species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Zootaxa 1902: 1-118.
- Eguchi K.; Bui T. V.; Yamane S. 2011. Generic synopsis of the Formicidae of Vietnam (Insecta: Hymenoptera), part I Myrmicinae and Pseudomyrmecinae. Zootaxa 2878: 1-61.
- Eguchi, K. 2001. A revision of the Bornean species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Tropics Monogr. Ser. 2: 1-154.
- Eguchi, K. 2004. Taxonomic revision of two wide-ranging Asian ants, Pheidole fervens and P. indica (Insecta: Hymenoptera, Formicidae) and related species. Ann. Naturhist. Mus. Wien. 105B:189-209.
- Eguchi, K. 2008. Eguchi, K. 2008. A revision of North Vietnamese species of the ant genus Pheidole. Zootaxa 1902: 1-118.
- Emery C. 1887. Catalogo delle formiche esistenti nelle collezioni del Museo Civico di Genova. Parte terza. Formiche della regione Indo-Malese e dell'Australia (continuazione e fine). [concl.]. Ann. Mus. Civ. Stor. Nat. 25(5): 427-473.
- Emery C. 1893. Formicides de l'Archipel Malais. Revue Suisse de Zoologie 1: 187-229.
- Emery C. Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei. Annali del Museo Civico di Storia Naturale 40: 661-722.
- Emery, C. "Catalogo delle formiche esistenti nelle collezioni del Museo Civico di Genova. Parte terza. Formiche della regione Indo-Malese e dell'Australia (continuazione e fine)." Annali del Museo Civico di Storia Naturale Giacomo Doria (Genova) (2) 5, no. 25 (1887): 427-473.
- Emery, C. "Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei." Annali del Museo Civico di Storia Naturale Giacomo Doria (Genova) (2) 20, no. 40 (1900): 661-722.
- Emery, C. "Voyage de MM. Bedot et Pictet dans l'Archipel Malais. Formicides de l'Archipel Malais." Revue Suisse de Zoologie 1 (1893): 187-229.
- Fischer G. & Fisher B. L. 2013. A revision of Pheidole Westwood (Hymenoptera: Formicidae) in the islands of the Southwest Indian Ocean and designation of a neotype for the invasive Pheidole megacephala. Zootaxa 3683: 301-356
- Fontanilla A. M., A. Nakamura, Z. Xu, M. Cao, R. L. Kitching, Y. Tang, and C. J. Burwell. 2019. Taxonomic and functional ant diversity along tropical, subtropical, and subalpine elevational transects in southwest China. Insects 10, 128; doi:10.3390/insects10050128
- Forel A. 1902. Les Formicides de l'Empire des Indes et de Ceylan. Part IX. J. Bombay Nat. Hist. Soc. 14: 520-546.
- Forel A. 1902. Myrmicinae nouveaux de l'Inde et de Ceylan. Rev. Suisse Zool. 10: 165-249.
- Forel A. 1905. Ameisen aus Java. Gesammelt von Prof. Karl Kraepelin 1904. Mitt. Naturhist. Mus. Hambg. 22: 1-26.
- Forel A. 1906. Les fourmis de l'Himalaya. Bulletin de la Société Vaudoise des Sciences Naturelles 42: 79-94.
- Forel A. 1912. Ameisen aus Java beobachtet und gesammelt von Edward Jacobson. III. Theil. Notes Leyden Mus. 34: 97-112
- Forel A. 1912. Einige neue und interessante Ameisenformen aus Sumatra etc. Zool. Jahrb. Suppl. 15: 51-78.
- Forel A. 1912. H. Sauter's Formosa-Ausbeute. Formicidae (Hym.) (Schluss). Entomol. Mitt. 1: 45-61.
- Forel A. 1913. H. Sauter's Formosa-Ausbeute: Formicidae II. Arch. Naturgesch. (A)79(6): 183-202
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Hu C.-H. 2006. Indigenized conservation and biodiversity maintenance on Orchid Island. PhD Thesis, graduate school of the University of Minnesota. 150 pages.
- Huang Jian-hua, Zhou Shan-yi. 2007. A checklist of family Formicidae of China - Myrmicinae (Part II) (Insecta: Hymenoptera). Journal of Guangxi Normal University : Natural Science Edition 25(1): 91-99.
- Huong N. T. T., P. V. Sang, and B. T. Viet. 2015. A preliminary study on diversity of ants (Hymenoptera: Formicidae) at Hon Ba Nature Reserve. Environmental Scientific Conference 7: 614-620.
- Jaitrong W., and T. Ting-Nga. 2005. Ant fauna of Peninsular Botanical Garden (Khao Chong), Trang Province, Southern Thailand (Hymenoptera: Formicidae). The Thailand Natural History Museum Journal 1(2): 137-147.
- Kutter H. 1932. Ameisen aus dem Museum zu Dresden. Mitt. Schweiz. Entomol. Ges. 15: 207-210.
- Leong C. M., S. F. Shiao, and B. Guenard. 2017. Ants in the city, a preliminary checklist of Formicidae (Hymenoptera) in Macau, one of the most heavily urbanized regions of the world. Asian Myrmecology 9: e009014.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Ogata K. 1982. Taxonomic study of the ant genus Pheidole Westwood of Japan, with a deszcription of a new species (Hymenoptera, Formicidae). Kontyu, Tokyo 50(2): 189-197.
- Pan Y.S. 2007. Systematic Study on the Ant Genera Pheidole Westwood and Aphaenogaster Mayr (Hymenoptera: Formincidae : Myrmicinae) In China. Guangxi Normal University, Guangxi, China. 73 pages.
- Pfeiffer M., D. Mezger, and J. Dyckmans. 2013. Trophic ecology of tropical leaf litter ants (Hymenoptera: Formicidae) - a stable isotope study in four types of Bornean rain forest. Myrmecological News 19: 31-41.
- Pfeiffer M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E. Y.; Kohout, R. J. 2011. The Formicidae of Borneo (Insecta: Hymenoptera): a preliminary species list. Asian Myrmecology 4:9-58
- Pfeiffer, M., H. Cheng Tuck, and T. Chong Lay. 2008. Exploring arboreal ant community composition and co-ccurrence patterns in plantations of oil palm Elaeis guineensis in Borneo and Peninsular Malaysia. Ecography 31(1): 21-32.
- Rizali A., M. M. Bos, D. Buchori, Sk. Yamane, and C. H. Schulze. 2008. Ants in tropical urban habitats: the myrmecofauna in a densely populated area of Bogor, West Java, Indonesia. Hayati Journal of Biosciences 15(2): 77-84.
- Rizali A., M.M. Bos, D. Buchori, Sk. Yamane, C. Hans, and J. Schulze. 2008. Ants in tropical urban habitats: the myrmecofauna in a densely populated area of Bogor, West Java, Indonesia. Hayati Journal of Biosciences 77-84.
- Santschi F. 1937. Fourmis du Japon et de Formose. Bulletin et Annales de la Société Entomologique de Belgique. 77: 361-388.
- Smith F. 1861. Catalogue of hymenopterous insects collected by Mr. A. R. Wallace in the islands of Ceram, Celebes, Ternate, and Gilolo. [part]. Journal and Proceedings of the Linnean Society of London. Zoology 6: 36-48.
- Smith, F. "Catalogue of the hymenopterous insects collected at Sarawak, Borneo; Mount Ophir, Malacca; and at Singapore, by A. R. Wallace." Journal of the Proceedings of the Linnean Society of London, Zoology 2 (1857): 42-88.
- Staab M., N. Bluthgen, and A. M. Klein. 2014. Tree diversity alters the structure of a tri-trophic network in a biodiversity experiment. Oikos doi: 10.1111/oik.01723
- Terayama M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta: Hymenoptera). Research Bulletin of Kanto Gakuen University. Liberal Arts 17:81-266.
- Terayama, M. 2009. A synopsis of the family Formicidae of Taiwan (Insecta; Hymenoptera). The Research Bulletin of Kanto Gakuen University 17: 81-266.
- Terayama. M. and Inoue. N. 1988. Ants collected by the members of the Soil Zoological Expedition to Taiwan. ARI Reports of the Myrmecologists Society (Japan) 18: 25-28
- Tian M., L. Deharveng, A. Bedos, Y. Li, Z. Xue, B. Feng, and G. Wei. 2011, Advances of cave biodiversity survey: a result based mainly on invertebrates. Proceedings of the 17th National Congress of Speleology, Yinshuidong, Hubei, 1-3 Nov 20111, p 149-163.
- Tiwari R.N., B.G. Kundu, S. Roychowdhury, S.N. Ghosh. 1999. Insecta: Hymenoptera: Formicidae. Pp. 211-294 in: Director; Zoological Survey of India (ed.) 1999. Fauna of West Bengal. Part 8. Insecta (Trichoptera, Thysanoptera, Neuroptera, Hymenoptera and Anoplura). Calcutta: Zoological Survey of India, iv + 442 pp.
- Way M. J., and B. Bolton. 1997. Competition between ants for coconut palm nesting sites. Journal of Natural History 31: 439-455.
- Wheeler W. M. 1909. Ants of Formosa and the Philippines. Bulletin of the American Museum of Natural History 26: 333-345.
- Wheeler W. M. 1919. The ants of Borneo. Bulletin of the Museum of Comparative Zoology 63:43-147.
- Wheeler W. M. 1927. Chinese ants collected by Professor S. F. Light and Professor N. Gist Gee. American Museum Novitates 255: 1-12.
- Wheeler W. M. 1929. Ants collected by Professor F. Silvestri in Formosa, the Malay Peninsula and the Philippines. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 24: 27-64.
- Wheeler W. M. 1929. Some ants from China and Manchuria. American Museum Novitates 361: 1-11.
- Wheeler W. M. 1930. A list of the known Chinese ants. Peking Natural History Bulletin 5: 53-81.
- Wheeler W. M. 1930. Formosan ants collected by Dr. R. Takahashi. Proceedings of the New England Zoological Club 11: 93-106.
- Yamane S. 2013. A Review of the ant fauna of the Krakatau Islands, Indonesia. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. Ser: A, 11: 1-66
- Yamane S.; Ikudome, S.; Terayama, M. 1999. Identification guide to the Aculeata of the Nansei Islands, Japan. Sapporo: Hokkaido University Press, xii + 831 pp. pp138-317.
- Zhang N. N., Y. Q. Chen, Z. X. Lu, W. Zhang, and K. L. Li. 2013. Species diversity, community structure difference and indicator species of leaf-litter ants in rubber plantations and secondary natural forests in Yunnan, southwestern China. Acta Entomologica Sinica 56(11): 1314-1323.
- Zhang R. J., L. W. Liang, and S. Y. Zhou. 2014. An analysis on the ant fauna of Nonggang Nature Reserve in Guangxi, China. Journal of Guangxi Normal university: Natural Science Edition 32(3): 86-93.
- Zhou S. Y., and Z. M. Zheng. 1999. Taxonomic study of the ant genus Pheidole Westwood from Guangxi, with descriptions of three new species (Hymenoptera: Formicidae). Acta Zootaxonomica Sinica 24: 83-88.
- Zhou S.-Y. and Zheng Z. 1999. Taxonomic study of the ant genus Pheidole Westwood from Guangxi, with descriptions of three new species (Hymenoptera: Formicidae). Acta Zootaxonomica Sinica24(1): 83-88.