Leptanilloides
| Leptanilloides | |
|---|---|
| Leptanilloides biconstrictus | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Dorylinae |
| Genus: | Leptanilloides Mann, 1923 |
| Type species | |
| Leptanilloides biconstrictus | |
| Diversity | |
| 19 species (Species Checklist, Species by Country) | |
| Synonyms | |
| |
This is a lineage of subterranean ants from Central and South America. Its members had been extremely rarely encountered before collecting methods targeting soil-dwelling ants were popularized: 17 out of the 19 currently known species were described after 1998. (Borowiec 2016)
| At a Glance | • Haiku |
Identification
Borowiec (2016) - Worker The workers of Leptanilloides are rather variable but can be distinguished from all other dorylines by a combination of promesonotal suture variously developed but often conspicuous, propodeal spiracles positioned low, lack of metanotal groove, absence of propodeal lobes, and small and unarmed pygidium. The positioning of the propodeal spiracle may be rather high on the sclerite in some species and so these could conceivably be mistaken for small Neivamyrmex. The latter, however, can be distinguished by a complete lack of a promesonotal suture in dorsal view and abdominal segment IV much larger than the succeeding segment V, with no apparent constrictions between abdominal segments IV, V, and VI. Although some larger Leptanilloides species can have an inconspicuous promesonotal suture, those will have visible constrictions on the gaster and abdominal segment IV and that segment is never much larger than succeeding gastral segments.
Male The males of Leptanilloides are distinct in their extreme reduction of tegulae. The wing venation is reduced and unusual among dorylines because of the combination of costal cell (C) present in fore wing and discal cell always being open. The males of Leptanilloides also lack conspicuously bispinose abdominal sternite IX (subgenital plate) characteristic of almost all other male dorylines.
Delsinne et al. (2015) provide the most recent key to species of Leptanilloides excluding Asphinctanilloides (see the link just below), but the species that were placed in the latter can still be identified using Brandão et al. (1999a): Key to Asphinctanilloides workers.
| See images of species within this genus |
Keys including this Genus
Keys to Species in this Genus
Distribution
Borowiec (2016) - Leptanilloides is a genus found throughout Central America, from Chiapas, Mexico to Panama, and from scattered, mostly high-elevation records from Bolivia, Colombia, Ecuador, and Venezuela. It is also present in the Amazon Basin and one species has been described from the Atlantic forest habitat of São Paulo, Brazil. A recent collection from western Texas (MacGown et al. 2015) is a remarkable range extension for this lineage.
Distribution and Richness based on AntMaps
Habitat
Based on reported specimen data, members of this genus group have been collected mostly in cloud forests (Borowiec & Longino, 2011; Brandão et al. 1999; Donoso et al. 2006; Longino 2003, Silva et al. 2013). Leptanilloides chihuahuaensis was collected in semiarid grassland habitat in the southern foothills of the Davis Mountains in western Texas, approximately 2500 km north of the next closest collection of the genus-group in Chiapas, Mexico.
Species by Region
Number of species within biogeographic regions, along with the total number of species for each region.
| Afrotropical Region | Australasian Region | Indo-Australian Region | Malagasy Region | Nearctic Region | Neotropical Region | Oriental Region | Palaearctic Region | |
|---|---|---|---|---|---|---|---|---|
| Species | 0 | 0 | 0 | 0 | 1 | 19 | 0 | 0 |
| Total Species | 2851 | 1736 | 3047 | 932 | 840 | 4391 | 1767 | 2925 |
Biology
Small hypogaeic blind ant-predators with subterranean legionary habits. Collecting techniques have undersampled ants that live an underground existence and this helps explain how little is known about these ants. Museums hold few Leptanilloides specimens. Label data shows many have been collected from cloud forests.
Anecdotal observations suggest these ants ("Leptanilloidinae" species) behave as army-ant like predators. These ants are likely to be largely subterranean, occasionally coming to the ground surface under debri (rocks, downed wood, etc.) while foraging or to allow mature sexuals (either males only or perhaps males and females) to leave their natal nest. Group predation, frequent colony migrations and synchronized brood cycles may also be part of their life history.
Borowiec (2016) - Leptanilloides nomadus was observed foraging at night in partly subterranean, partly exposed, columns but the ants did not carry any prey (Donoso et al. 2006). In Leptanilloides nubecula the colonies are apparently polygynous, with subdichthadiigyne queens and intercastes present (Donoso et al. 2006), but a complete colony of Leptanilloides erinys contained only single subdichthadiiform queen (Borowiec and Longino 2011). Brood apparently develops in synchrony, as all nest collections so far contain larvae of uniform size (Brandão et al. 1999a,b, Donoso et al. 2006, Borowiec and Longino 2011). Brandão et al. (1999b) summarized the scant information available on species then classified in Asphinctanilloides (=Leptanilloides). They report workers of Leptanilloides anae moving in columns similar to that of army ants, preying on an unidentified, dismembered arthropod under cow dung. This would suggest that this species may not be a specialized predator of other ants, like most dorylines, although it is clear that more observations are needed. As evidence for hypogaeic habits Brandão et al. (1999b) note that where intensive surveys of leaf litter ants have been carried out specimens have not been collected by that method but only from soil samples. Specimens have also been extracted from stomachs of subterranean amphisbaenians, giving further evidence for the underground lifestyle.
Brandão et al. (1999) - Leptanilloides is exclusively Neotropical, recorded from the Andean foothills at 440 m in Bolivia and in higher altitudes (> 3200 m and up) in Colombia and Ecuador. The biology is completely unknown, except some indication of army ant life style by the study of Leptanilloides legionarius larvae. The strong girdling constrictions separating the three visible gastral segments is unique among leptanilloidine ants and a similar situation is only found in the cerapachyine Sphinctomyrmex, which has the armed pygidium not reduced. Although the larvae we have in hand are not well preserved, we found nothing similar to the larval haemolymph structure described by Masuko (1989) in Leptanilla japonica.
Donoso et al. (2006) - The army ant syndrome is defined as a combination of three discrete features: nomadism, obligate group-predation and queen dichthadiigyny. Brandão, Silva et al. (1999) provided indirect evidence that army-ant like behavior occurs in the subfamily Leptanilloidinae (=Dorylinae). First, they observed synchrony in larva production, which suggests nomadism. Second, two observations suggested group predatory behavior: 1) direct observation of group-predation by an Leptanilloides anae colony on an arthropod and 2) presence on larvae of mandibles with slender, sharp pointed teeth that facilitate being carried by workers. One of the worker paratypes of Leptanilloides legionarius Brandão et al. was preserved holding a larva in the fashion typical of army ants, with the larva slung ventrally under the body of the worker (Figure 6 in Brandão, Diniz et al. 1999), and our field observations confirm this behavior in Leptanilloides nomadus and Leptanilloides nubecula. Our observations provide further evidence for synchrony in brood production, as noted by the presence of numerous (>100) larvae in a single growth stage during collections of L. nomadus and L. nubecula. Also, our observations demonstrate for Leptanilloides subterranean habits, trail-following behavior, and nocturnal activity.
The discovery of the L. nubecula gyne with a subdichtadiigyne habitus brings new data to the discussion of army ant origins. The leptanilloidine queen differs from true army ants in at least two respects: the gaster is not fully physogastric (maybe due to the relatively small number of workers in a normal leptanilloidine colony, or to a particular colony stage when collected), and the presence of eyes. However, in common with true army ants, leptanilloidine gynes are apterous and have an enlarged postpetiole and falcate mandibles. These characteristics suggest obligate fission as the reproductive strategy for Leptanilloides (see discussion in Gotwald 1995; Peeters & Ito 2001). Workers also show variable expression of typical army ant characters. Unlike true army ants, the colonies are not extraordinarily large, and there is no extreme polymorphism like some of the army ants. The Leptanilloidinae suggest a scenario for what the early stages of army ant evolution might have been.
The queen polymorphism (one true gyne plus several ergatogynes) found in L. nubecula is not new in the dorylomorphs (see Peeters & Ito 2001). Polygyny was reported for Neivamyrmex carolinensis (Rettenmeyer & Watkins 1978). In this species, polygyny was presumed to be correlated with special ecological limitations imposed by the tough environment in which the ants live. They occur at the northern limit of the range of army ants, and there may be a high rate of gyne death due to cold winters (Gotwald 1995). The cold conditions of the high Andean cloud forest may similarly favor polygyny in Leptanilloides.
The new species L. nomadus and L. nubecula share strikingly similar character states with Leptanilloides mckennae and Leptanilloides legionarius with respect to external morphology. Shared characters include: 1) high reduction or lack of genal teeth, 2) petiolar form intermediate between Asphinctanilloides and Leptanilloides, 3) postpetiole smaller than petiole, 4) postpetiolar spiracle shifted backwards on anteromedian side of tergite, and 5) blunt flange over metapleural gland opening. These characters blur the distinction between Leptanilloides and Asphinctanilloides, an observation previously noted by Longino (2003) and Ward (2006). It is noteworthy that the males of the two species for which males are known differ greatly in mandible shape. The mandible of L. nubecula males are falcate while those of L. mckennae are subtriangular. The presence of sickle-shaped mandibles in L. nubecula males provides additional evidence that at least some Leptanilloides show trends toward stereotypical army ant morphology (see Ward 2006).
The discovery of three Leptanilloides species living in sympatry provides evidence of considerably more ecological diversity than was previously thought. The different Leptanilloides species may well be occupying distinct subterranean ecological niches. True army ants usually also occur as diverse communities of sympatric species (Gotwald 1995), although in that group as well little is known about how the different species partition local resources It is unknown whether these sympatric Leptanilloides speciated in sympatry or whether they co-occur as a result of secondary contact. In any case, the Otonga reserve and other Andean cloud forests are of extremely high importance for the study and conservation of Leptanilloides ants, a significance noted more generally by Myers et al. (2000) and documented for mammals (Jarrin 2001) and vascular plants (Jaramillo 2001).
Castes
Worker
Morphology
Worker Morphology
 Explore: Show all Worker Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Worker Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
Male Morphology
 Explore: Show all Male Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Male Morphology data or Search these data. See also a list of all data tables or learn how data is managed.
• Caste unknown
Phylogeny
| Dorylinae |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See Phylogeny of Dorylinae for details.
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- LEPTANILLOIDES [Leptanilloidinae]
- Leptanilloides Mann, 1923: 13. Type-species: Leptanilloides biconstrictus, by original designation.
- AMYRMEX [junior synonym of Leptanilloides]
- Amyrmex Kusnezov, 1953b: 333. Type-species: Amyrmex golbachi, by original designation.
- Amyrmex junior synonym of Forelius: Shattuck, 1992c: 87.
- Amyrmex revived from synonymy: Cuezzo, 2000: 271.
- Amyrmex in Leptanilloidinae: Ward & Brady, 2009: 48.
- Amyrmex junior synonym of Leptanilloides: Borowiec, 2016: 150.
- ASPHINCTANILLOIDES [junior synonym of Leptanilloides]
- Asphinctanilloides Brandão, Diniz, Agosti & Delabie, 1999: 30. Type-species: Asphinctanilloides anae, by original designation.
- Asphinctanilloides junior synonym of Leptanilloides: Borowiec, 2016: 150.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Taxonomic Notes
Borowiec (2016) - Leptanilloides was described with one species, Leptanilloides biconstrictus, in 1923 (Mann 1923). Subsequently a closely related genus Asphinctanilloides was described, along with new Leptanilloides species (Brandão et al. 1999a). Asphinctanilloides was originally differentiated from Leptanilloides by several characters. The genus Amyrmex, known only from males, was originally placed erroneously in the Dolichoderinae (Ward and Brady 2009). Recent descriptions of additional Leptanilloides species reveal greater morphological diversity of the genus and blur the distinction from Asphinctanilloides (Longino 2003, Donoso et al. 2006, Borowiec and Longino 2011). Because of this it has been suggested that Leptanilloides may eventually prove paraphyletic with regards to Asphinctanilloides and it has already been shown to be paraphyletic with respect to Amyrmex (see Ward and Brady 2009). No Asphinctanilloides have ever been included in a molecular analysis but it is possible that the males described as Amyrmex in fact correspond to Asphinctanilloides. Here I propose synonymy of both Amyrmex and Asphinctanilloides under Leptanilloides. Leptanilloides in this new sense is easily identified in both worker and males and guarantees more stable taxonomy in the face of potential paraphyly issues, although it encompasses morphologically disparate forms. It is possible that future workers will feel justified to split this genus once again after a better understanding of worker and male diversity is attained.
The phylogenetic position of Leptanilloides within Dorylinae was difficult to ascertain with Sanger sequencing data (Brady et al. 2014) but genomic data suggest that it forms a clade with Sphinctomyrmex and New World army ants. Several species have been included in morphology-based and molecular phylogenies (Brandão et al. 1999a, Ward 2007, Ward and Brady 2009, Delsinne et al. 2015, MacGown et al. 2015, Borowiec, in prep.) but the availability of fresh material precludes a comprehensive analysis.
McGown et al. (2015) - Of the 16 species in the Leptanilloides genus-group, males of only three have been given species names, and of those three, only males of Leptanilloides nubecula and Leptanilloides mckennae were associated with their respective worker castes (Donosa et al. 2006,Ward 2007). The description of Leptanilloides golbachi was based solely on the male caste (Kusnezov 1953). Three other male morphotypes were described by Borowiec & Longino (2011), but, because they were unassociated with workers, they could not be certain of their specific identities. Therefore, Borowiec & Longino (2011) simply referred to these male morphotypes as Leptanilloidinae male 1, male 2, and male 3. Borowiec & Longino (2011) stated that “Leptanilloidinae male 1” was likely conspecific with Leptanilloides gracilis based on various shared morphological features, the relatively small size, and the largely sympatric distribution of “Leptanilloidinae male 1” and workers of L. gracilis. The status of Leptanilloidinae males 2 and 3 is less clear. These male morphotypes might represent the males of described species in the group or they may be new species. While searching unidentified material in the Bohart Museum of Entomology collection (UCDC), Ward & Brady (2009) discovered several male Leptanilloides genus-group males that represent at least five more species in the group. These appeared to belong to Amyrmex and possibly Asphinctanilloides, although it is not clear whether they represented new species or were the males of described species in the group.
Description
Worker
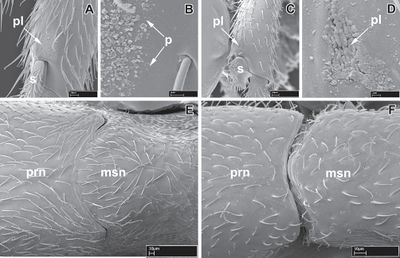
Borowiec (2016) - Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent or present. Parafrontal ridges absent or reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum without median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2- or, rarely, 1-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth or triangular with median tooth. Eyes absent. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent or present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection variable, with suture present, weakly differentiated, immobile or with suture conspicuous and complete, but immobile, or with suture complete and mobile. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove weakly impressed to deeply impressed and conspicuous. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity absent. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated low on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal suture placed at posttergite and axial or slightly supraaxial. Prora simple, not delimited by carina, a simple U-shaped margin, or a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III variable, more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist) or abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured or a gradual concavity, not gutter-like. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent or present. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent or present. Pygidium small, reduced to narrow strip, without impressed medial field and simple, not armed with cuticular spines or modified setae. Hypopygium unarmed. Legs: Mid tibia with single simple/barbulate spur, with single pectinate spur, or rarely with two simple spurs. Hind tibia with pectinate spur or rarely with one barbulate and one pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Queen
Borowiec (2016) - The queens of Leptanilloides collected so far have been ‘subdichthadiiform’, or wingless ergatoids with eyes and hypertrophied gaster, including abdominal segment III. The gynes possess eyes but no ocelli. See description of Leptanilloides erinys gyne; Donoso et al. (2006) also reported blind intercastes in addition to a subdichthadiigyne in Leptanilloides nubecula.
Male
Head: Antennae with 13 segments. Clypeus with or without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps unknown. Labial palps unknown. Mandibles falcate or, more rarely, elongately triangular, edentate, or intermediate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal suture placed at posttergite or at suture and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX simple or cleft, with lateral apodemes reduced, only medial apodeme conspicuous, short. Genitalia: Cupula short relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, with sulcus discernable at junction or no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally compressed,
rounded at apex. Legs: Mid tibia with single simple/barbulate spur or with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula absent or extremely small. Vein C in fore wing present. Pterostigma narrow or broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M or absent. Abscissa M·f4 in fore wing absent or present, not reaching wing margin. Cross-vein 1m-cu in fore wing absent. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissa A·f1 present. Vein C in hind wing absent or present. Vein R in hind wing absent. Vein Sc+R in hind wing absent or present. Abscissa Rs·f1 in hind wing absent or present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing absent or present, not reaching wing margin. Cross-vein 1rs-m in hind wing absent. Vein M+Cu in hind wing absent. Abscissa M·f1 in hind wing absent. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent. Vein Cu in hind wing absent. Vein A in hind wing absent.
Larva
Brandão et al. (1999) - (description based on larva of Leptanilloides legionarius). First 5 abdominal segments clearly differentiated from another; posterior somites indistinct (Fig. 48). Long (50 μm) unbranched, smooth, slightly curved hairs, and a continuous covering of very small (less than 10 μm) unbranched, smooth, slightly curved to flexuous hairs. Head longer than wide (Fig. 49); genae bulging and frons depressed (Fig. 50); antennae relatively long, almost as long as mandibles, with 2 apical sensillae; labrum circular or heart-shaped in face view, elongate. Mandibles without striae or spinules, their outer border furnished with 6 slender sharp-pointed teeth; apical and subapical teeth longer than remaining (Figs 49, 51). Maxillae without paxiliform or digitiform palp, with apical sensillae.
Borowiec (2016) - Presence of cocoons unknown.
References
- Baroni Urbani, C.; Bolton, B.; Ward, P. S. 1992. The internal phylogeny of ants (Hymenoptera: Formicidae). Syst. Entomol. 17: 301-329 (page 317, Leptanilloides in Leptanilloidinae)
- Bolton, B. 1990a. Abdominal characters and status of the cerapachyine ants (Hymenoptera, Formicidae). J. Nat. Hist. 24: 53-68 (page 61, Leptanilloides in Cerapachyinae, Cerapachyini)
- Bolton, B. 1990e. Army ants reassessed: the phylogeny and classification of the doryline section (Hymenoptera, Formicidae). J. Nat. Hist. 2 24: 1339-1364 (page 1357, Leptanilloides in Cerapachyinae, Cerapachyini)
- Bolton, B. 1994. Identification guide to the ant genera of the world. Cambridge, Mass.: Harvard University Press, 222 pp. (page 71, Leptanilloides in Leptanilloidinae)
- Bolton, B. 2003. Synopsis and Classification of Formicidae. Mem. Am. Entomol. Inst. 71: 370pp (page 146, Leptanilloides in Leptanilloidinae)
- Borgmeier, T. 1955. Die Wanderameisen der neotropischen Region. Stud. Entomol. 3: 1-720 (page 653, Leptanilloides incertae sedis in Formicidae)
- Borowiec, M. L.; Longino, J. T. 2011. Three new species and reassessment of the rare Neotropical ant genus Leptanilloides (Hymenoptera, Formicidae, Leptanilloidinae). ZooKeys 133:19-48. [2011-10-05]
- Borowiec, M.L. 2016. Generic revision of the ant subfamily Dorylinae (Hymenoptera, Formicidae). ZooKeys 608: 1-280 (doi:10.3897/zookeys.608.9427).
- Borowiec, M.L. 2019. Convergent evolution of the army ant syndrome and congruence in big-data phylogenetics. Systematic Biology 68, 642–656 (doi:10.1093/sysbio/syy088).
- Boudinot, B.E. 2019. Hormigas de Colombia. Cap. 15. Clave para las subfamilias y generos basada en machos. Pp. 487-499 in: Fernández, F., Guerrero, R.J., Delsinne, T. (eds.) 2019d. Hormigas de Colombia. Bogotá: Universidad Nacional de Colombia, 1198 pp.
- Brandão, C. R. F.; Diniz, J. L. M.; Agosti, D.; Delabie, J. H. C. 1999. Revision of the Neotropical ant subfamily Leptanilloidinae. Syst. Entomol. 24: 17-36 (page 23, Leptanilloides in Leptanilloidinae)
- Brown, W. L., Jr. 1973b. A comparison of the Hylean and Congo-West African rain forest ant faunas. Pp. 161-185 in: Meggers, B. J., Ayensu, E. S., Duckworth, W. D. (eds.) Tropical forest ecosystems in Africa and South America: a comparative review. Washington, D.C.: Smithsonian Institution Press, viii + 350 pp. (page 166, Leptanilloides in Ecitoninae)
- Brown, W. L., Jr. 1975. Contributions toward a reclassification of the Formicidae. V. Ponerinae, tribes Platythyreini, Cerapachyini, Cylindromyrmecini, Acanthostichini, and Aenictogitini. Search Agric. (Ithaca N. Y.) 5(1 1: 1-115 (page 18, Leptanilloides in Ponerinae, Cerapachyini)
- Cantone S. 2018. Winged Ants, The queen. Dichotomous key to genera of winged female ants in the World. The Wings of Ants: morphological and systematic relationships (self-published).
- Dlussky, G. M.; Fedoseeva, E. B. 1988. Origin and early stages of evolution in ants. Pp. 70-144 in: Ponomarenko, A. G. (ed.) Cretaceous biocenotic crisis and insect evolution. Moskva: Nauka, 232 pp. (page 79, Leptanilloides in Ponerinae, Cerapachyini)
- Donisthorpe, H. 1943g. A list of the type-species of the genera and subgenera of the Formicidae. [part]. Ann. Mag. Nat. Hist. 11(10): 617-688 (page 656, Leptanilloides in Dorylinae, Ecitonini)
- Donoso, D.A., Vieira, J.M. & Wild, A.L. 2006. Three new species of Leptanilloides from Andean Ecuador. Zootaxa 1201: 47-62.
- Fernandez, F., Guerrero, R.J., Sánchez-Restrepo, A.F. 2021. Sistemática y diversidad de las hormigas neotropicales. Revista Colombiana de Entomología 47, 1–20 (doi:10.25100/socolen.v47i1.11082).
- Hölldobler, B.; Wilson, E. O. 1990. The ants. Cambridge, Mass.: Harvard University Press, xii + 732 pp. (page 10, Leptanilloides in Ponerinae, Cerapachyini)
- Jaffe, K. 1993. El mundo de las hormigas. Baruta, Venezuela: Equinoccio (Ediciones de la Universidad Simón Bolívar), 188 pp. (page 9, Leptanilloides in Cerapachyinae, Cerapachyini)
- Kempf, W. W. 1972b. Catálogo abreviado das formigas da regia~o Neotropical. Stud. Entomol. 15: 3-344 (page 129, Leptanilloides incertae sedis in Dorylinae)
- MacGown, J.A., Schiefer, T.L. & Branstetter, M.G. 2015. First record of the genus Leptanilloides (Hymenoptera: Formicidae: Dorylinae) from the United States. Zootaxa. 4006:392–400.
- Mann, W. M. 1923. Two new ants from Bolivia. (Results of the Mulford Biological Exploration. - Entomology.). Psyche (Camb.) 30: 13-18 (page 13, Leptanilloides in Dorylinae)
- Silva, R.R., Feitosa, R.M., Brandao, C.R.F. & Freitas, A.V.L. 2013. The first Leptanilloides species (Hymenoptera: Formicidae: Leptanilloidinae) from eastern South America. Journal of Natural History (http://dx.doi.org/10.1080/00222933.2012.763058).
- Wheeler, G. C.; Wheeler, J. 1985b. A simplified conspectus of the Formicidae. Trans. Am. Entomol. Soc. 111: 255-264 (page 259, Leptanilloides incertae sedis in Formicidae)