Strumigenys talpa
| Strumigenys talpa | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Strumigenys |
| Species group: | talpa |
| Species: | S. talpa |
| Binomial name | |
| Strumigenys talpa Weber, 1934 | |
| Synonyms | |
| |
This species is among the most commonly collected Nearctic species. It occurs from Florida to Washington, D.C., and extends west to Oklahoma and Texas (Brown 1950). Strumigenys talpa tolerates a wide variety of mesic to dry habitats and is less common in wet habitats (Deyrup 2016). Unlike the wide-ranging species Strumigenys missouriensis, Strumigenys talpa exhibits little morphological variation across its range. However, there appears to be a smooth morphological transition over geographic space between S. talpa and Strumigenys deyrupi (a junior synonym of S. talpa). Setae on the anterior margin of the scape are typically directed towards the apex of the scape in S. talpa but some setae are directed towards the base of the scape in specimens collected in Florida from where S. deyrupi was described. Mark Deyrup (for whom S. deyrupi was named), has also come to this realization and we believe S. deyrupi to either be the same species or to hybridize heavily where their ranges overlap (southern half of Georgia into Florida). I have not observed scape setae variation anywhere else across the range of S. talpa except in specimens from Texas and there is no evidence of discrete forms in sympatry. All other setae characters of the Texas specimens are congruent with S. talpa. Texas specimens all have flagellate setae on their mesosoma, pronotal shoulders, abdominal tergites, and femur/tibia. Also unusual are the Oklahoma workers that lack elongate apicoscrobal setae. This character is uniformly present in all other collections across S. talpa’s range that I have examined including those from Texas. Although these setae could have been abraded, this is unlikely as the specimens examined were in excellent shape and had no missing or broken flagellate setae on other portions of the body. I expect the Oklahoma specimens represent a new species but am grouping them with S. talpa until more specimens are found and S. talpa is examined across its range. (Booher, 2021)
Identification
Bolton (2000) - A member of the Strumigenys talpa-group. As Brown (l953a) has pointed out, the three older species currently placed in this group are very closely related, and best separated by differences in their clypeal pilosity. Strumigenys wrayi has hairs on the anterior clypeal margin that curve away from the midline and spoon-shaped hairs on the lateral margins that are weakly reflexed; hairs on the clypeal dorsum are inclined posteriorly. Clypeal hairs with these orientations are absent from Strumigenys filitalpa and talpa. In particular the fringe of hairs on the lateral margins curves anteriorly; those of talpa being decidedly spatulate or weakly spoon-shaped whilst those of filitalpa are slender and filiform. Neither has posteriorly inclined hairs on the clypeal dorsum.
Pyramica deyrupi
Bolton (2000) - A member of the Strumigenys pulchella-group. In addition to the comparative features noted under Strumigenys creightoni, Strumigenys deyrupi differs from Strumigenys metazytes as the latter has distinctly smaller and narrower spongiform lobes on the waist segments. As well as the character noted in the key, the petiole of metazytes in profile has the maximum length of the lateral lobe less than half the length of the node; in deyrupi this lobe is distinctly greater than half the node length. The size difference in clypeal pilosity between the hairs located mid-dorsally and those that fringe the lateral margins is obviously different in deyrupi and Strumigenys abdita, and easily discerned even under low magnification.
P. deyrupi bears a striking resemblance to Strumigenys talpa, but in the latter species all the hairs on the leading edge of the scape are curved toward the scape apex.
Keys including this Species
- Key to Nearctic Strumigenys (as Pyramica)
- Key to US Strumigenys species
- Key to western Nearctic Strumigenys species
Distribution
USA; in eastern USA from Florida to New Jersey and west to Missouri; in western USA occurs in Oklahoma and Texas (Booher, 2021).
Latitudinal Distribution Pattern
Latitudinal Range: 39.991° to 27.18333333°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
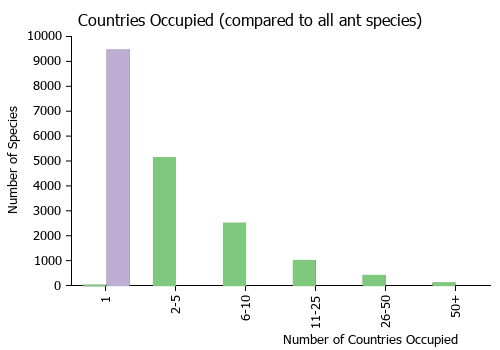
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
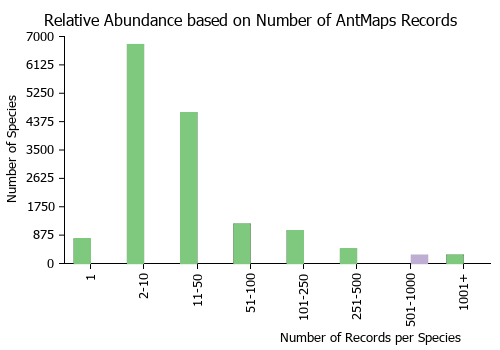
|
Biology
Wesson and Wesson (1939) from their description of the synonymized S. venatrix: described from a colony containing about 60 workers. Eight colonies and occasional scattered workers have been taken in Pike, Lawrence, Scioto and Adams Counties. The species is definitely a soil or humus dweller and forages for Collembola under the leaves and dead vegetable matter on the surface of the ground. So far as we can tell, it is not associated with other species for the purpose of obtaining the Collembola about their nest. Specific examples of the colonies may give a better idea of the habitus. A colony was found in a small opening near the edge of some young oak woods on a rather dry, gently-sloping hillside. The soil was a sandy clay. Several workers were first observed around a light cover of dead leaves. One of these, carrying a springtail in its mandibles, led to the nest, the entrance of which, was a tiny hole under a flake of stone in the middle of a small bare area 30 sq. cm. in extent. Just below the surface, this hole widened out into a spacious, elongate chamber 5 to 10 mm. in diameter and 10 cm. in length, which appeared to be the hollow interior of a dead and decayed root. Another colony was found in the grassy humus on the edge of a bushy thicket in a field. A colony of Aphaenogaster fulva was under an adjacent stone. Four colonies, including the type, were found in a grassy clearing in some dry, open woods. Two of these colonies were on the surface in the tangled roots of the grass, while the other 2 were in the soil 2 to 8 cm. below the surface. Galleries of Camponotus americanus ran close to one nest, but we were unable to find any connection between the two. Two colonies were 2ound in the cedar thicket described above under Strumigenys missouriensis. One of these was nesting in an opening at the bottom of the humus, the other in a small cavity at the base of an old rotted cedar stump. Stray workers in these and other places were often found by pulling back the top cover of the humus in places where springtails were abundant.
Castes
Worker
Images from AntWeb
  
| |
| Worker. Specimen code casent0102557. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by MHNG, Geneva, Switzerland. |
   
| |
| Paratype of Strumigenys venatrix. Worker. Specimen code casent0103123. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by USNM, Washington, DC, USA. |
  
| |
| Worker. Specimen code casent0104490. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
   
| |
| Worker. Specimen code casent0103172. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0104488. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
Male
Images from AntWeb
   
| |
| Worker. Specimen code casent0104489. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
    
| |
| Paratype of Strumigenys venatrix. Male (alate). Specimen code casent0103124. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by USNM, Washington, DC, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- talpa. Strumigenys (Cephaloxys) talpa Weber, 1934b: 63, fig. 1 (w.) U.S.A. Brown, 1953g: 77 (q.m.); Wheeler, G.C. & Wheeler, J. 1955a: 141 (l.). Combination in S. (Trichoscapa): Smith, M.R., 1947f: 587; Creighton, 1950a: 310; in Smithistruma: Smith, M.R., 1951a: 828; Brown, 1953g: 76; in Pyramica: Bolton, 1999: 1673; in Strumigenys: Baroni Urbani & De Andrade, 2007: 128. Senior synonym of venatrix: Wesson, 1949: 21. See also: Wilson, 1954: 486; Bolton, 2000: 132.
- deyrupi. Pyramica deyrupi Bolton, 2000: 119 (w.) U.S.A.
- Combination in Strumigenys: Baroni Urbani & De Andrade, 2007: 118.
- Status as species: Deyrup, 2003: 46.
- Junior synonym of talpa: Deyrup, 2017: 140.
- venatrix. Strumigenys (Cephaloxys) venatrix Wesson, L.G. & Wesson, R.G., 1939: 103, pl. 3, fig. 5 (w.) U.S.A. Combination in S. (Trichoscapa): Smith, M.R., 1943f: 307. Junior synonym of talpa: Wesson, 1949: 21; Brown, 1953g: 76.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
TL 2.0-2.2, HL 0.53-0.60, HW 0.36-0.39, CI 64-69, ML 0.08-0.11, MI 15-19, SL 0.25-0.29, SI 66-76, PW 0.23-0.26, AL 0.50-0.59 (20 measured).
Fully closed mandibles with a basal gap between anterior clypeal margin and basal tooth that is distinctly longer than length of basal tooth. Anterior clypeal margin very evenly and shallowly broadly convex. In full-face view lateral clypeal margins with a continuous fringe of distinctly projecting, anteriorly curved hairs that are spatulate to very feebly spoon-shaped. Row of hairs on clypeal dorsum closest to lateral margin also project outward and curve anteriorly above the principal row; these hairs shorter than the principal marginal hairs but longer than those situated more centrally on the dorsum. Anterior clypeal margin without hairs that curve away from the midline. Dorsum of clypeus densely clothed with small spatulate hairs that curve anteriorly or anterolaterally. Dorsolateral margin of head with a single projecting flagellate hair, in apicoscrobal position. Dorsum of head close to occipital margin with 1-2 pairs of flagellate hairs. Flagellate hairs also present at pronotal humeri, on pronotal dorsum and mesonotum (1 pair each), on first gastral tergite and dorsal (outer) surfaces of hind tibia and basitarsus. Basigastral costulae strongly developed, extending one-third or more the length of the tergite.
Pyramica deyrupi
Holotype. TL 1.9, HL 0.52, HW 0.33, CI 63, ML 0.09, MI 17, SL 0.26, SI 79, PW 0.22, AL 0.48. Anterior clypeal margin almost transverse in full-face view. Dorsum of clypeus with numerous small short spatulate hairs that are somewhat elevated and curved anteriorly; these hairs tiny by comparison with the long, laterally projecting, anteriorly curved spatulate to narrowly spoon-shaped hairs that fringe the lateral clypeal margins. Dorsolateral margin of head with a flagellate apicoscrobal hair. Cephalic ground pilosity curved and narrowly spatulate, elevated; vertex near occipital margin with a pair of longer erect flagellate hairs. Pronotal humeral hair very long and fine, flagellate. Pronotal dorsum with 1 pair of erect flagellate hairs; mesonotum with a pair of flagellate hairs. Hairs on first gastral tergite very fin e and flagellate ; similar hairs present on waist segments. Hind basitarsus with a fine flagellate hair projecting from the dorsal (outer) surface close to its base. Basigastral costulae coarse, strongly developed , extending over the basal quarter of the tergite.
Paratypes. TL 1.9-2.0, HL 0.52-0.54, HW 0.33-0.35, CI 63-65, ML 0.09-0.10, MI 17-19, SL 0.26-0.28, SI 78-81, PW 0.22-0.24, AL 0.48-0.50 (5 measured).
Type Material
Holotype worker, U. S. A. Illinois, Herod, 12.x.1933 (T.H. Frison & H.H. Ross) (Illinois Natural History Survey Insect Collection) [not seen].
Pyramica deyrupi
Holotype worker, U.S.A.: Florida, Ocala, 9 mi. SSW Marion Co., 16.x.1990, Ocala waterway, scrub area (M. Deyrup) (Museum of Comparative Zoology) [holotype is top specimen on a pin of three].
Paratypes. All specimens from same series (absolute number not known) (Museum of Comparative Zoology, Archhold Biological Station Florida, UTEL, The Natural History Museum)
References
- Baroni Urbani, C. and De Andrade, M.L. 2007. The ant tribe Dacetini: limits and constituent genera, with descriptions of new species. Annali del Museo Civico di Storia Naturale “G. Doria”. 99:1-191.
- Bolton, B. 1999. Ant genera of the tribe Dacetonini (Hymenoptera: Formicidae). J. Nat. Hist. 3 33: 1639-1689 (page 1673, Combination in Pyramica)
- Bolton, B. 2000. The ant tribe Dacetini. Memoirs of the American Entomological Institute. 65:1-1028. (page 132, catalogue)
- Booher, D.B. 2021. The ant genus Strumigenys Smith, 1860 (Hymenoptera: Formicidae) in western North America north of Mexico. Zootaxa 5061, 201–248 (doi:10.11646/zootaxa.5061.2.1).
- Brown, W. L., Jr. 1953g. Revisionary studies in the ant tribe Dacetini. American Midland Naturalist. 50:1-137. (page 77, queen, male described; page 76, Combination in Smithistruma)
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Gochnour, B.M., Suiter, D.R., Booher, D. 2019. Ant (Hymenoptera: Formicidae) fauna of the Marine Port of Savannah, Garden City, Georgia (USA). Journal of Entomological Science 54, 417-429 (doi:10.18474/jes18-132).
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87 (doi:10.3897@jhr.70.35207).
- Weber, N. A. 1934b. A new Strumigenys from Illinois (Hymenoptera: Formicidae). Psyche (Camb.) 41: 63-65 (page 63, fig. 1 worker described)
- Wesson, L. G. 1949. Strumigenys venatrix Wesson and Wesson synonymous with S. talpa Weber. Psyche (Camb.) 56: 21 (page 21, senior synonym of venatrix)
- Wesson, L. G.; Wesson, R. G. 1939. Notes on Strumigenys from southern Ohio, with descriptions of six new species. Psyche. 46:91-112.
- Wheeler, G. C.; Wheeler, J. 1955c. The ant larvae of the myrmicine tribe Solenopsidini. Am. Midl. Nat. 54: 119-141 (page 141, larva described)
- Wilson, E. O. 1954a [1953]. The ecology of some North American dacetine ants. Ann. Entomol. Soc. Am. 46: 479-495 (page 486, biology)
References based on Global Ant Biodiversity Informatics
- Annotated Ant Species List Ordway-Swisher Biological Station. Downloaded at http://ordway-swisher.ufl.edu/species/os-hymenoptera.htm on 5th Oct 2010.
- Brown W. L., Jr. 1953. Revisionary studies in the ant tribe Dacetini. Am. Midl. Nat. 50: 1-137.
- Carroll T. M. 2011. The ants of Indiana (Hymenoptera: Formicidae). Master's Thesis Purdue university, 385 pages.
- Colby, D. and D. Prowell. 2006. Ants (Hymenoptera: Formicidae) in Wet Longleaf Pine Savannas in Louisiana. Florida Entomologist 89(2):266-269
- Coovert G. A. 2005. The Ants of Ohio (Hymenoptera: Formicidae). Ohio Biological Survey, Inc. 15(2): 1-207.
- Coovert, G.A. 2005. The Ants of Ohio (Hymenoptera: Formicidae) Ohio Biological Survey Bulletin New Series Volume 15(2):1-196
- Dash S. T. and L. M. Hooper-Bui. 2008. Species diversity of ants (Hymenoptera: Formicidae) in Louisiana. Conservation Biology and Biodiversity. 101: 1056-1066
- Deyrup M., C. Johnson, G. C. Wheeler, J. Wheeler. 1989. A preliminary list of the ants of Florida. Florida Entomologist 72: 91-101
- Deyrup, M. 2003. An updated list of Florida ants (Hymenoptera: Formicidae). Florida Entomologist 86(1):43-48.
- Deyrup, M. and J. Trager. 1986. Ants of the Archbold Biological Station, Highlands County, Florida (Hymenoptera: Formicidae). Florida Entomologist 69(1):206-228
- Deyrup, M. and S. Cover. 2009. Dacetine Ants in Southeastern North America (Hymenoptera: Formicidae). Southeastern Naturalist 8(2):191-212
- Dubois, M.B. and W.E. Laberge. 1988. An Annotated list of the ants of Illionois. pages 133-156 in Advances in Myrmecology, J. Trager
- Forster J.A. 2005. The Ants (hymenoptera: Formicidae) of Alabama. Master of Science, Auburn University. 242 pages.
- Frye J. A., T. Frye, and T. W. Suman. 2014. The ant fauna of inland sand dune communities in Worcester County, Maryland. Northeastern Naturalist, 21(3): 446-471.
- Hill J.G. & Brown R. L. 2010. The Ant (Hymenoptera: Formicidae) Fauna of Black Belt Prairie Remnants in Alabama and Mississippi. Southeastern Naturalist. 9: 73-84
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87.
- Ivanov K., L. Hightower, S. T. Dash, and J. B. Keiper. 2019. 150 years in the making: first comprehensive list of the ants (Hymenoptera: Formicidae) of Virginia, USA. Zootaxa 4554 (2): 532–560.
- Johnson C. 1986. A north Florida ant fauna (Hymenoptera: Formicidae). Insecta Mundi 1: 243-246
- Lubertazzi D. and Tschinkel WR. 2003. Ant community change across a ground vegetation gradient in north Floridas longleaf pine flatwoods. 17pp. Journal of Insect Science. 3:21
- MacGown J. A., J. G. Hill, R. L. Brown, T. L. Schiefer, J. G. Lewis. 2012. Ant diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi. Mississippi Agricultural and Forestry Experiment Station Bulletin 1197: 1-30
- MacGown J. A., J. G. Hill, and M. Deyrup. 2009. Ants (Hymenoptera: Formicidae) of the Little Ohoopee River Dunes, Emanuel County, Georgia. J. Entomol. Sci. 44(3): 193-197.
- MacGown J. A., J. G. Hill, and R. L. Brown. 2010. Native and exotic ant in Mississippi state parks. Proceedings: Imported Fire Ant Conference, Charleston, South Carolina, March 24-26, 2008: 74-80.
- MacGown J. A., and R. L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A and J.A. Forster. 2005. A preliminary list of the ants (Hymenoptera: Formicidae) of Alabama, U.S.A. Entomological News 116(2):61-74
- MacGown, J.A. and JV.G. Hill. Ants of the Great Smoky Mountains National Park (Tennessee and North Carolina).
- MacGown, J.A. and R.L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A., J.G. Hill, R.L. Brown and T.L. 2009. Ant Diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi Report #2009-01. Schiefer. 2009.
- MacGown, J.A., R.L. Brown and J.G. Hill. 2005. An Annotated List of the Pyramica (Hymenoptera: Formicidae: Dacetini) of Mississippi. Journal of the Kansas Entomological Societ 78 (3):285-289
- Macgown J. A., S. Y. Wang, J. G. Hill, and R. J. Whitehouse. 2017. A List of Ants (Hymenoptera: Formicidae) Collected During the 2017 William H. Cross Expedition to the Ouachita Mountains of Arkansas with New State Records. Transactions of the American Entomological Society, 143(4): 735-740.
- Van Pelt A., and J. B. Gentry. 1985. The ants (Hymenoptera: Formicidae) of the Savannah River Plant, South Carolina. Dept. Energy, Savannah River Ecology Lab., Aiken, SC., Report SRO-NERP-14, 56 p.
- Weber N. A. 1934. A new Strumigenys from Illinois (Hymenoptera: Formicidae). Psyche (Camb.) 41: 63-65
- Wesson L. G., and R. G. Wesson. 1939. Notes on Strumigenys from southern Ohio, with descriptions of six new species. Psyche (Cambridge) 46: 91-112.

