Stenamma impar
| Stenamma impar | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Stenammini |
| Genus: | Stenamma |
| Species: | S. impar |
| Binomial name | |
| Stenamma impar Forel, 1901 | |
Smith (1957) reports: In her population studies of soil nesting ants in the Droste Woods, St.Charles County, Missouri, from September to March during the years 1948 to 1953, Miss Mary Talbot found Stenamma impar to be the most common Stenamma, Stenamma schmittii second, and Stenamma meridionale third. It is surprising that no individuals of Stenamma brevicorne were taken. She is positive that in many instances she did not collect entire colonies of S. impar. Colonies or portions of colonies were collected at depths from 4 to 16 inches. Usually only one chamber was found, occasionally there were two. The largest number of workers collected from a colony was 109, the least 5. Only one mother queen was found per colony in 4 colonies. It appears from these and other records that most if not all colonies have only a single mother queen. Miss Talbot found that it is common for the larvae to overwinter in the nest; however, a half dozen or less eggs were found in 2 colonies as late as the middle of October. At the time of collection colonies were taken from both dry and damp soils. In the Edwin S. George Reserve in Livingston County, Michigan, Miss Talbot collected winged females and males from a colony on August 13, 1949. In the same locality K. Bohnsack collected 33 workers, 3 alate females and 1 male from a colony on August 19, 1949. Although Miss Talbot commonly found the species nesting in the soil, one of our records may indicate that impar also nests in rotten or faulty wood. This species has been collected at altitudes up to 4760 feet.
Photo Gallery
Identification
Smith 1957: The worker of Stenamma impar can in general be distinguished by its small size, slender form, fineness of body sculpturing, and usually light brown or yellowish brown color. Other important characters are: The rather small, coarsely faceted eye which measures approximately 0.10 mm in its greatest diameter and contains 5-6 ommatidia; funicular segments 2 through 7 broader than long; distinct to very pronounced mesoepinotal impression; the distinct but short, tuberculate spines which are angularly borne on the mesosoma; the very strikingly angular petiolar node (in profile); and the weakly developed longitudinal rugulae at the base of the gaster.
Smith cautions that worker size and color can be quite variable.
Distribution
Massachusetts to Georgia, west to North Dakota, Illinois, and Missouri (Smith 1957).
Latitudinal Distribution Pattern
Latitudinal Range: 45.567° to 33.1675°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
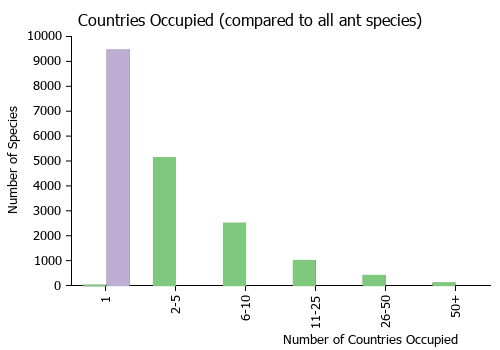
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
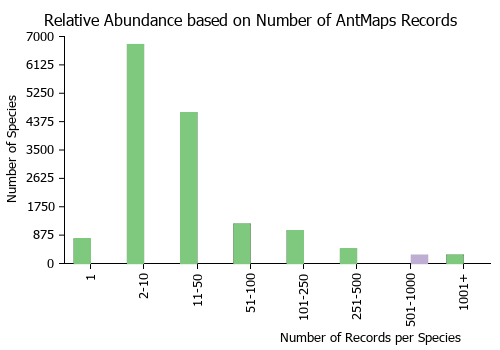
|
Biology
Talbot (1957) published an account of nests found by excavating soil during the cooler months of the year in a Missouri woodland: "Workers were easy to see, for although they were small they were bright colored, were clustered in well defined chambers in firm clay soil and moved slowly when disturbed. Eight collections of complete colonies averaged 108 individuals, of which half were workers. Larvae were overwintered, and a few eggs were still present in November. Chambers were fairly deep in the soil: one was four inches below the surface, but the others ranged from seven to sixteen inches with a mean depth of 10.1 inches. A typical chamber was 1/2 inch long, 3/8 inch wide, and 1/4 inch high, dome-shaped and very smooth. Usually a colony occupied only one chamber."
Castes
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- impar. Stenamma brevicorne r. impar Forel, 1901e: 347 (w.q.) U.S.A. (Virginia, Massachusetts).
- Type-material: lectotype worker (by designation of Smith, M.R. 1957b: 152), paralectotype workers (number not stated), 1 paralectotype queen.
- Type-locality: lectotype U.S.A.: nr Washington D.C., Virginia bank of Potomac River (T. Pergande, and A. Forel) (by restriction of Smith, M.R. 1957b: 152); paralectotype workers with same data.
- [Note: other paralectotype locality: 1 queen, U.S.A.: Massachusetts, Boston, Franklin Park (A. Forel).]
- Type-depositories: MHNG (lectotype); AMNH, MHNG, USNM (paralectotypes).
- Smith, M.R. 1957b: 152 (m.).
- Subspecies of brevicorne: Wheeler, W.M. 1903d: 167 (in key); Wheeler, W.M. 1910g: 565; Emery, 1921f: 54; Smith, M.R. 1951a: 795.
- Status as species: Creighton, 1950a: 137; Smith, M.R. 1957b: 150 (redescription); Smith, M.R. 1958c: 116; Smith, M.R. 1967: 352; Francoeur, 1977b: 206; Smith, D.R. 1979: 1359; Allred, 1982: 506; DuBois & LaBerge, 1988: 143; Wheeler, G.C., et al. 1994: 304; Bolton, 1995b: 393; Coovert, 2005: 42; Ellison, et al. 2012: 321.
- Distribution: Canada, U.S.A.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Smith (1957) - Length 2.3-2.7 mm. Funicular segments 2 through 7 broader than long; last segment of antennal club approximately as long as the combined lengths of the three preceding segments. Eye subelliptical, approximately 0.10 mm in its greatest diameter and with 5-6 ommatidia; all ommatidia distinct and coarse in appearance. Thorax, in profile, with distinct to frequently pronounced mesoepinotal impression, the impression sometimes as much as 0.10 mm long and about half as deep. Base of epinotum meeting the declivity to form a pair of short, but distinct, variable sized, tuberculate spines which are borne on the epinotum as pronounced angles. Petiolar node, in profile, very distinctly angular; viewed from above and behind, the node is slender (often with dorsally converging sides), compressed antero-posteriorly, with truncate or weakly rounded superior border. Postpetiolar node, from above, almost as long as broad and with a subrectangular to subglobular appearance; the anterior two-thirds of the dorsum convex and somewhat anteroposteriorly compressed. Base of gaster bearing weakly developed, longitudinal rugulae which are usually 0.10 mm or less in length.
Frontal region of head with fine, posteriorly diverging, longitudinal striae which are scarcely discernible in some lights. Mandible longitudinally rugulose, with scattered, coarse punctures. Cheeks largely composed of longitudinal rugulae. Most of head bearing fine, rugulose-reticulate sculpture in which the interspaces are punctulate. The sculpturing of the head not always clearly defined because of the nature of the color of the head and the fineness of the sculpture. Thorax above, with a fine sculpture which largely ranges from longitudinally rugulose to rugulose-reticulate, that on the promesonotum usually of a longitudinally rugulose nature. Petiolar and postpetiolar nodes largely finely punctulate except for their dorsal surfaces which are usually shining, especially in certain lights. Frontal area and gaster smooth and shining and also much of the propleuron.
Body usually light brown or yellowish brown, occasionally dark brown; gaster however usuallly with an infuscated, transverse band near the middle.
The worker may vary in size and color as noted above. The gaster occasionally lacks the infliscated band. The mesoepinotal impression varies from distinct to very pronounced. The epinotal spines although always distinct and tuberculate range from small and fine to moderately large and coarse. The petiolar node, when viewed from above and behind, may have sides, which are subparallel or dorsally converging and the superior border of the node may vary from subtruncate to weakly rounded. The postpetiolar node, from above, although subrectangular to subglobular in appearance varies to some extent in proportion but is apparently slightly wider than long. The sculpturing of the body seems to be most variable on the thorax, especially on the epinotum, where it is often irregular although more commonly reticulate or rugulose-reticulate.
Queen
Smith (1957) - Similar to the worker except as described below.
Length 3.2-3.5 mm. Antennal scape lacking its greatest diameter or less of attaining the posterior border of the head. Ocelli small, pale, the anterior ocellus usually located about 0.3 mm posterior to the frontal area. Eye oblong, usually 0.2 mm in its greatest diameter and with 10-12 ommatidia. Thorax from 1.2-1.3 mm in length measured from the anterior border of the pronotal collar to the apices of the epinotal spines, widest just anterior to the wing articulations where it measures from 0.6-0.65 mm. From above, the humeri are subangular. Anterior wings subopaquish, with light brown or yellowish veins and stigma; venation similar to that of brevicorne. Base of epinotum, in profile, meeting the declivity in a very broad angle. Epinotal spines highly variable in size and shape, usually ranging from 0.05-0.10 mm in length. Petiole, in profile, pedunculate with very distinctly angular node. Postpetiole, in profile, scarcely longer than high, convex above and highest near its midlength. Petiolar node, from above and behind, usually with dorsally converging sides and weakly rounded or subtruncate superior' border.
Mesonotum highly variable in sculpturing, ranging from smooth and shining in appearance with fine longitudinal rugulae and punctate interspaces to subopaque with coarser sculpture. The longitudinal rugulae on the scutellum commonly coarser and more apparent than on the mesonotum. Posterior part of epinotum, above, between, and below the epinotal spines often smooth and shining, occasionally finely sculptured above the spines. Propleuron largely longitudinally rugulose as are also the side of the epinotum, the latter however with more distinctly punctulate interspaces. Mesopleuron, especially the meso-episternum, often largely smooth and shining. Petiole and postpetiole punctulate or rugulose-punctulate except for the rather smooth and shining nodes. Hairs moderately abundant, slender, light yellowish, reclinate or suberect for the most part.
Body light brown or yellowish brown to dark reddish brown, with lighter appendages, eyes black, mandibular teeth and often the scutellum and wing articulations dark. Gaster. highly variable in color, the apex usually light and the remainder of the gaster light brown to almost blackish, occasionally the gaster bears a dark, transverse band.
As the above description indicates, the female is subject to considerable variation, especially in color and sculpture. The variation in sculpturing is especially noticeable on the head, mesonotum and mesopleuron. The length and width of the thorax seems to be fairly constant in the small number of specimens studied. I have not seen any individuals in which the base of the epinotum and declivity form a single plane as Forel has described.
Male
Smith (1957) - Length 2.9 mm. Head narrower in front of the eyes than behind the eyes, 0.35 mm in width. Posterior border of the eye approximately 0.25 mm anterior to the posterior border of the head. Eye large, convex, protuberant; from in front, usually more than 0.2 mm in length and 0.10 mm in width. Anterior ocellus located approximately 0.4 mm back of the anterior border of the clypeus. Clypeus convex above, the carinae vestigial or absent, the anterior border arched. Mandible slender, the masticatory border usually with three or four teeth, the first and second apical being the most distinct. Antennal scape (exclusive of the pedicel) approximately 0.3 mm in length and equal to the combined lengths of the first three or four funicular segments. Thorax from above, 0.9 mm in length from the anterior border of the pronotal collar to the apex of the scutellum and 0.65-0.7 mm in width just anterior to the articulations of the wings. Mesonotal outline forming a broad arch anteriorly. Mayrian furrows distinct. In profile, base of epinotum meeting the declivity in a rounded angle; epinotal spines lacking, represented only by scarcely perceptible angular ridges. Petiole, in profile, pedunculate, with angular node. Petiolar and postpetiolar nodes, from above, rather narrow. Petiolar node, from behind, with subparallel sides and transversely truncate superior border. Postpetiolar node, from above, approximately as broad as long and of a subrectangular appearance.
Most of head finely punctulate, scutellum very finely longitudinally rugulose, side of epinotum irregularly sculptured, with the punctulation apparently dominating. Mandibles, much of mesonotum and mesopleuron, dorsum of petiolar and postpetiolar nodes, and gaster, smooth and shining.
Pilosity moderately abundant, slender, largely suberect or reclinate.
Body sordid light brown with lighter appendages.
The most notable variations were the length of the body, which ranged from 2.6-2.9 mm; the scape length 2.66-3 mm; length of thorax 0.8-0.9 mm; and width anterior to the articulations of the wings from 0.6-0.7 mm; eye, from in front, usually more than 0.2 mm in length, occasionally less, and usually more than 0.10 mm in width, occasionally less; color of body ranging from a pale, sordid brown to a much darker, sordid brown; wings subopaquish to hyaline. One individual had an unusually small discoidal cell in one wing and none in the other.
Type Material
From Smith (1957): Described from workers collected by A. Forel and Theodore Pergande on the Virginia bank of the Potomac River near Washington, D. C., while sifting damp leaves; also from a dealate female unassociated with other castes collected by Forel in Franklin Park, Boston, Massachusetts. I hereby designate Virginia as the type locality and have selected as a lectotype, a cotype worker from the Museum d'Histoire Naturelle Geneva, Switzerland. Types located: Museum d'Histoire Naturelle, Geneva, Switzerland American Museum of Natural History, U. S. National Museum.
Etymology
Morphological. impar L. = unequal. Forel was perhaps referring to the short and uneven spines found on the propodeum.
References
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Chick, L.D., Lessard, J.-P., Dunn, R.R., Sanders, N.J. 2020. The coupled influence of thermal physiology and biotic interactions on the distribution and density of ant species along an elevational gradient. Diversity 12, 456 (doi:10.3390/d12120456).
- Creighton, W. S. 1950a. The ants of North America. Bulletin of the Museum of Comparative Zoology 104: 1-585 (page 137, raised to species)
- Fairweather, A.D., Lewis, J.H., Hunt, L., Smith, M.A., McAlpine, D.F. 2020. Ants (Hymenoptera: Formicidae) of Rockwood Park, New Brunswick: An assessment of species richness and habitat. Northwestern Naturalist 27(3):576–584.
- Forel, A. 1901j. Variétés myrmécologiques. Ann. Soc. Entomol. Belg. 45: 334-382 (page 347, worker, queen described)
- Ipser, R.M., Brinkman, M.A., Gardner, W.A., Peeler, H.B. 2004. A survey of ground-dwelling ants (Hymenoptera: Formicidae) in Georgia. Florida Entomologist 87: 253-260.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87 (doi:10.3897@jhr.70.35207).
- MacGown, J.A., Booher, D., Richter, H., Wetterer, J.K., Hill, J.G. 2021. An updated list of ants of Alabama (Hymenoptera: Formicidae) with new state records. Transactions of the American Entomological Society 147: 961-981 (doi:10.3157/061.147.0409).
- Smith, M. R. 1957b. Revision of the genus Stenamma Westwood in America north of Mexico (Hymenoptera, Formicidae). Am. Midl. Nat. 57: 133-174 (page 152, ?)
- Talbot, M. 1957. Populations of ants in a Missouri woodland. Insectes Sociaux. 4:375-384.
- Waters, J.S., Keough, N.W., Burt, J., Eckel, J.D., Hutchinson, T., Ewanchuk, J., Rock, M., Markert, J.A., Axen, H.J., Gregg, D. 2022. Survey of ants (Hymenoptera, Formicidae) in the city of Providence (Rhode Island, United States) and a new northern-most record for Brachyponera chinensis (Emery, 1895). Check List 18(6), 1347–1368 (doi:10.15560/18.6.1347).
References based on Global Ant Biodiversity Informatics
- Carroll T. M. 2011. The ants of Indiana (Hymenoptera: Formicidae). Master's Thesis Purdue university, 385 pages.
- Clark A. T., J. J. Rykken, and B. D. Farrell. 2011. The Effects of Biogeography on Ant Diversity and Activity on the Boston Harbor Islands, Massachusetts, U.S.A. PloS One 6(11): 1-13.
- Coovert G. A. 2005. The Ants of Ohio (Hymenoptera: Formicidae). Ohio Biological Survey, Inc. 15(2): 1-207.
- Coovert, G.A. 2005. The Ants of Ohio (Hymenoptera: Formicidae) Ohio Biological Survey Bulletin New Series Volume 15(2):1-196
- Del Toro, I. 2010. PERSONAL COMMUNICATION. MUSEUM RECORDS COLLATED BY ISRAEL DEL TORO
- Dubois, M.B. and W.E. Laberge. 1988. An Annotated list of the ants of Illionois. pages 133-156 in Advances in Myrmecology, J. Trager
- Ellison A. M., J. Chen, D. Díaz, C. Kammerer-Burnham, and M. Lau. 2005. Changes in ant community structure and composition associated with hemlock decline in New England. Pages 280-289 in B. Onken and R. Reardon, editors. Proceedings of the 3rd Symposium on Hemlock Woolly Adelgid in the Eastern United States. US Department of Agriculture - US Forest Service - Forest Health Technology Enterprise Team, Morgantown, West Virginia.
- Ellison A. M., S. Record, A. Arguello, and N. J. Gotelli. 2007. Rapid Inventory of the Ant Assemblage in a Temperate Hardwood Forest: Species Composition and Assessment of Sampling Methods. Environ. Entomol. 36(4): 766-775.
- Ellison A. M., and E. J. Farnsworth. 2014. Targeted sampling increases knowledge and improves estimates of ant species richness in Rhode Island. Northeastern Naturalist 21(1): NENHC-13NENHC-24.
- Francoeur A. 2010. Liste des especes de fourmis (Formicides, Hymenopteres). Entomofaune du Quebec. Document Faunique no. 1, Version 5, 0. 1-10 pp.
- Frye J. A., T. Frye, and T. W. Suman. 2014. The ant fauna of inland sand dune communities in Worcester County, Maryland. Northeastern Naturalist, 21(3): 446-471.
- General D. M., and L. C. Thompson. 2011. New Distributional Records of Ants in Arkansas for 2009 and 2010 with Comments on Previous Records. Journal of the Arkansas Academy of Science 65: 166-168.
- Gotelli, N.J. and A.M. Ellison. 2002. Biogeography at a Regional Scale: Determinants of Ant Species Density in New England Bogs and Forests. Ecology 83(6):1604-1609
- Heithaus R. E., and M. Humes. 2003. Variation in Communities of Seed-Dispersing Ants in Habitats with Different Disturbance in Knox County, Ohio. OHIO J. SCI. 103 (4): 89-97.
- Ipser R. M. 2004. Native and exotic ants (Hymenoptera: Formicidae) of Georgia: Ecological Relationships with implications for development of biologically-based management strategies. Doctor of Philosophy thesis, University of Georgia. 165 pages.
- Ivanov, K. 2019. The ants of Ohio (Hymenoptera, Formicidae): an updated checklist. Journal of Hymenoptera Research 70: 65–87.
- Ivanov K., L. Hightower, S. T. Dash, and J. B. Keiper. 2019. 150 years in the making: first comprehensive list of the ants (Hymenoptera: Formicidae) of Virginia, USA. Zootaxa 4554 (2): 532–560.
- Kjar D. 2009. The ant community of a riparian forest in the Dyke Marsh Preserve, Fairfax County, Virginiam and a checklist of Mid-Atlantic Formicidae. Banisteria 33: 3-17.
- Kjar D., and E. M. Barrows. 2004. Arthropod community heterogeneity in a mid-Atlantic forest highly invaded by alien organisms. Banisteria 23: 26-37.
- Kjar D., and Z. Park. 2016. Increased ant (Hymenoptera: Formicidae) incidence and richness are associated with alien plant cover in a small mid-Atlantic riparian forest. Myrmecological News 22: 109-117.
- Lynch J. F. 1981. Seasonal, successional, and vertical segregation in a Maryland ant community. Oikos 37: 183-198.
- Lynch J. F. 1988. An annotated checklist and key to the species of ants (Hymenoptera: Formicidae) of the Chesapeake Bay region. The Maryland Naturalist 31: 61-106
- Lynch J. F., and A. K. Johnson. 1988. Spatial and temporal variation in the abundance and diversity of ants (Hymenoptera: Formicidae) in the soild and litter layers of a Maryland forest. American Midland Naturalist 119(1): 31-44.
- MacGown J. A., J. G. Hill, R. L. Brown, T. L. Schiefer, J. G. Lewis. 2012. Ant diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi. Mississippi Agricultural and Forestry Experiment Station Bulletin 1197: 1-30
- MacGown, J.A. and JV.G. Hill. Ants of the Great Smoky Mountains National Park (Tennessee and North Carolina).
- MacGown, J.A. and R.L. Brown. 2006. Survey of the ants (Hymenoptera: Formicidae) of the Tombigbee National Forest in Mississippi. Journal of the Kansas Entomological Society 79(4):325-340.
- MacGown, J.A., J.G. Hill, R.L. Brown and T.L. 2009. Ant Diversity at Noxubee National Wildlife Refuge in Oktibbeha, Noxubee, and Winston Counties, Mississippi Report #2009-01. Schiefer. 2009.
- Mahon M. B., K. U. Campbell, and T. O. Crist. 2017. Effectiveness of Winkler litter extraction and pitfall traps in sampling ant communities and functional groups in a temperate forest. Environmental Entomology 46(3): 470–479.
- Menke S. B., E. Gaulke, A. Hamel, and N. Vachter. 2015. The effects of restoration age and prescribed burns on grassland ant community structure. Environmental Entomology http://dx.doi.org/10.1093/ee/nvv110
- O'Neill J.C. and Dowling A.P.G. 2011. A Survey of the Ants (hymenoptera: Formicidae) of Arkansas and the Ozark Mountains. An Undergraduate Honors, University of Arkansas. 18pages.
- Shik, J., A. Francoeur and C. Buddle. 2005. The effect of human activity on ant species (Hymenoptera: Formicidae) richness at the Mont St. Hilaire Biosphere Reserve, Quebec. Canadian Field-Naturalist 119(1): 38-42.
- Smith M. R. 1957. Revision of the genus Stenamma Westwood in America north of Mexico (Hymenoptera, Formicidae). American Midland Naturalist 57: 133-174.
- Talbot M. 1957. Populations of ants in a Missouri woodland. Insectes Sociaux 4(4): 375-384.
- Talbot M. 1976. A list of the ants (Hymenoptera: Formicidae) of the Edwin S. George Reserve, Livingston County, Michigan. Great Lakes Entomologist 8: 245-246.
- Wheeler G. C., J. N. Wheeler, and P. B. Kannowski. 1994. Checklist of the ants of Michigan (Hymenoptera: Formicidae). The Great Lakes Entomologist 26(4): 297-310
- Wheeler, G.C., J. Wheeler and P.B. Kannowski. 1994. CHECKLIST OF THE ANTS OF MICHIGAN (HYMENOPTERA: FORMICIDAE). Great Lakes Entomologist 26:1:297-310


