Pheidole annexa
| Pheidole annexa | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Pheidole |
| Species: | P. annexa |
| Binomial name | |
| Pheidole annexa Eguchi, 2001 | |
This inhabits well-developed forests. I have never encountered colonies which include more than one dealate queen. (Eguchi 2001)
Identification
Eguchi (2001) - This medium-sized species with general habitus is distinguished from other Indo-Malayan congeners by a combination of the following characteristics: hypostoma of the major bearing three poorly developed median processes of which each lateral one is partly combined with the process just mesal to mandibular insertion; posterior declivity of promesonotal dome having a prominence in both the subcastes; petiole 0.9-1.0 times as long as postpetiole in both the subcastes; promesonotum of the minor unarmed without any kind of tubercle.
Keys including this Species
- Key to Pheidole majors and minors of Borneo
- Key to Pheidole majors of Borneo
- Key to Pheidole minors of Borneo
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: 5.033333333° to 4°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Indo-Australian Region: Borneo (type locality), Indonesia, Malaysia, Philippines.
Oriental Region: Thailand.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Castes
Minor
Images from AntWeb
   
| |
| Paratype of Pheidole annexa. Worker. Specimen code casent0901626. Photographer Ryan Perry, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- annexa. Pheidole annexus Eguchi, 2001b: 32, fig. 6 (s.w.q.) BORNEO.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
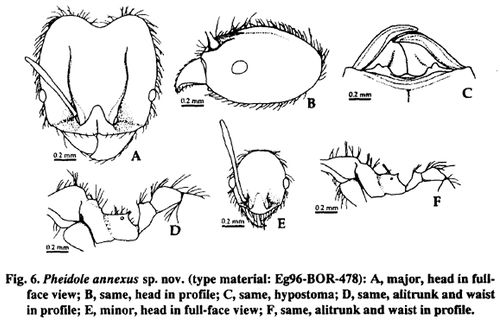
Major (n=5): TL 2.4-2.7 mm, HL 1.08-1.22 mm, HW 1.03-1.16 mm, SL 0.64-0.78 mm, FL 0.85-1.03 mm, CI 95-96, SI 60-67, FI 81-89. Head broadest at about 1/2-3/5 distance of head (as measured from the mid-point of a transverse line spanning the anteriormost and posteriormost projecting points, respectively) (Fig. 6A), in profile not impressed on vertex (Fig. 6B). Hypostoma with three poorly developed median processes, of which each lateral one is partly combined with the process just mesal to mandibular base (Fig. 6C). Clypeus with a weak median longitudinal carina, with anterior margin emarginate medially. Eye situated at about 1/3 distance of head; distance between mandibular insertion and anterior margin of eye 1.5-1.7 times as long as maximal diameter of eye. Frontal carina extending backward to 3/4 distance of head. Antennal scrobe inconspicuous, running along frontal carina. Antenna with 3-segmented club; scape extending backward to about 3/4 distance of head; terminal segment ca. 0.9 times as long as preceding two segments together. Masticatory margin of mandible with apical and preapical teeth, and a denticle in front of basal angle. Promesonotal dome with a distinct prominence on its posterior declivity (Fig. 6D); the prominence in anterior view not or very weakly concave medially. Mesopleuron without a distinct transverse impression. Propodeal spine horn-like, straight or slightly curved, 2.5-3 times as long as diameter of propodeal spiracle. Petiole cuneiform, 0.9-1.0 times as long as postpetiole (excluding helcium) (Fig. 6D); petiolar node low, in posterior view not or hardly emarginate at apex. Postpetiole in dorsal view subpentagonal, 2.3-2.4 times as broad as petiolar node.
Frons and area between mandibular insertion and eye longitudinally rugose; area between antennal scrobe and subocular level, vertex, and dorsal and lateral faces of occipital lobe rugosoreticulate; dorsum of promesonotum with transverse rugulae but shining; lateral face of promesonotum weakly rugose or rugoso-reticulate; mesopleuron and lateral face of propodeum very weakly punctured, or partly smooth and shining; lateral faces of petiolar pedicel and postpetiole weakly or very weakly punctured; dorsa of petiole and postpetiole, and gaster smooth and shining. Outer face of mandible covered with relatively long decumbent hairs, which are 0.06-0.13 mm in length and (a little) longer than distance between piligerous punctures. Body brown with a little darker gaster; legs sometimes a little lighter than alitrunk.
Minor (n=5): TL 1.8-2.1 mm, HL 0.56-0.64 mm, HW 0.48-0.53 mm, SL 0.70-0.82 mm, AL 0.80-0.91 mm, FL 0.80-0.93 mm, CI 81-85, SI 147-162, Fl168-183. Head in full-face view oval (Fig. 6E), with distinct occipital carina. Clypeus without a median longitudinal carina, with anterior margin in full-face view truncate, or slightly concave medially. Eyes situated just in front of mid length of head; distance between mandibular insertion and anterior margin of eye 1.1-1.2 times as long as maximal diameter of eye. Frontal carina and antennal scrobe present only around antennal insertion. Antenna with 3-segmented club; in full-face view scape extending beyond posterior border of head by more than its 1/3 length; terminal segment ca. 0.8 times as long as preceding two segments together. Promesonotal dome with a low prominence on its posterior declivity (Fig. 6F). Mesopleuron without a distinct transverse impression. Propodeal spine small, elongate-triangular, almost twice as long as diameter of propodeal spiracle. Petiole cuneiform, 0.9-1.0 times as long as postpetiole (excluding helcium) (Fig. 6F); petiolar node low, in posterior view not emarginate at apex. Postpetiole in dorsal view subpentagonal, 2.0-2.3 times as broad as petiolar node.
Head and promesonotum smooth and shining; mesopleuron and lateral face of propodeum almost smooth and shining, or very weakly punctured partly; lateral face of petiolar pedicel very weakly punctured: dorsum of petiole, and postpetiole and gaster smooth and shining. Body light yellowish-brown.
Type Material
Holotype Major, colony: Eg96-BOR-478, Sepilok forest, Sabah, E. Malaysia (Borneo), K. Eguchi leg., 1998, deposited in Universiti Malaysia Sabah. Paratypes 5 majors and 6 minors from the same colony to which the holotype belongs, deposited in The Natural History Museum, Museum of Comparative Zoology, Naturhistorisches Museum Wien, Vienna and UMS.
References
- Eguchi, K. 2001a. A revision of the Bornean species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Tropics Monograph Series. 2:1-154.
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
References based on Global Ant Biodiversity Informatics
- Berghoff S.M., U. Maschwitz, and K.E. Linsemair. 2003. Hypogaeic and epigaeic ant diversity on Borneo: evaluation of baited sieve buckets as a study method. Tropical Zoology 16: 153-163.
- Bruhl C.A. 2001. Leaf litter ant communities in tropical lowland rain forests in Sabah, Malaysia: effects of forest disturbance and fragmentation. PHD thesis Wurzburg Universitat, 168 pp.
- Eguchi K. 2001. A revision of the Bornean species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Tropics Monograph Series 2: 1-154.
- Eguchi K., and S. Yamane. 2003. Species diversity of ants (Hymenoptera, Formicidae) in a lowland rainforest, northwestern Borneo. New Entomol. 52(1,2): 49-59.
- Fayle T. M., E. C. Turner, J. L. Snaddon, V. Khen Chey, A. Y. C. Chung, P. Eggleton, and W. A. Foster. 2010. Oil palm expansion into rain forest greatly reduces ant biodiversity in canopy, epiphytes and leaf-litter. Basic and Applied Ecology 11: 337345.
- Jaitrong W.; Nabhitabhata, J. 2005. A list of known ant species of Thailand. The Thailand Natural History Museum Journal 1(1): 9-54.
- Mezger D., and M. Pfeiffer. 2011. Partitioning the impact of abiotic factors and spatial patterns on species richness and community structure of ground ant assemblages in four Bornean rainforests. Ecography 34: 39-48.
- Mezger D., and M. Pfeiffer. 2011. Partitioning the impact of abiotic factors and spatial patterns on species richness and community structure of ground assemblages in four Bornean rainforest. Ecography 34: 39-48.
- Mustafa N.A., H.M.W. Salim, C. Fletcher, A.R. Kassim, and M.D. Potts. 2011. Taxonomic and functional diversity of ants (Hymenoptera: Formicidae) in an upper hill dipterocarp forest in Peninsular Malaysia. The Raffles bulletin of Zoology 59(2): 181-194.
- Pfeiffer M., D. Mezger, and J. Dyckmans. 2013. Trophic ecology of tropical leaf litter ants (Hymenoptera: Formicidae) - a stable isotope study in four types of Bornean rain forest. Myrmecological News 19: 31-41.
- Pfeiffer M., and D. Mezger. 2012. Biodiversity Assessment in Incomplete Inventories: Leaf Litter Ant Communities in Several Types of Bornean Rain Forest. PLoS ONE 7(7): e40729. doi:10.1371/journal.pone.0040753
- Pfeiffer M., and D. Mezger. 2012. Biodiversity Assessment in Incomplete Inventories: Leaf Litter Ant Communities in Several Types of Bornean Rain Forest. PLoS ONE 7(7): e40729. doi:10.1371/journal.pone.0040950
- Pfeiffer M., and D. Mezger. 2012. Biodiversity Assessment in Incomplete Inventories: Leaf Litter Ant Communities in Several Types of Bornean Rain Forest. PLoS ONE 7(7): e40729. doi:10.1371/journal.pone.0041059
- Pfeiffer, M., H. Cheng Tuck, and T. Chong Lay. 2008. Exploring arboreal ant community composition and co-ccurrence patterns in plantations of oil palm Elaeis guineensis in Borneo and Peninsular Malaysia. Ecography 31(1): 21-32.
- Woodcock P., D. P. Edwards, R. J. Newton, C. Vun Khen, S. H. Bottrell, and K. C. Hamer. 2013. Impacts of Intensive Logging on the Trophic Organisation of Ant Communities in a Biodiversity Hotspot. PLoS ONE 8(4): e60756. doi:10.1371/journal.pone.0060756
- Woodcock P., D. P. Edwards, T. M. Fayle, R. J. Newton, C. Vun Khen, S. H. Bottrell, and K. C. Hamer. 2011. The conservation value of South East Asia's highly degraded forests: evidence from leaf-litter ants. Phil. Trans. R. Soc. B. 366: 3256-3264.