Anochetus graeffei
| Anochetus graeffei | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Anochetus |
| Species: | A. graeffei |
| Binomial name | |
| Anochetus graeffei Mayr, 1870 | |
| Synonyms | |
| |
Nests are generally under rocks or occasionally other objects on the ground but Anochetus graeffei is also known to nest directly in soil without covering, in termite nests and in rotten wood. This is one of the most widely distributed species within the genus, occurring from southern India east through SE Asia to Australia and onwards to the Cook Islands; it is also one of the most morphologically variable.
| At a Glance | • Invasive |
Photo Gallery
Identification
Shattuck and Slipinska (2012) - Australia: Eyes very small (EL < 0.16mm); front of head with sculpturing extending to the posterior margin; pronotum with coarse and heavy striate-rugose sculpture; body very hairy, with abundant erect hairs on all surfaces. This is the most heavily sculptured species of Anochetus within Australia, approached only by Anochetus victoriae. These two taxa can be separated by the rugulose rather than striate sculpturing on the dorsum of the pronotum and the smaller eye (eye length < 0.16mm vs. > 0.22mm) in A. graeffei.
Zettel (2012) - Philippines: workers and gyne examined are peculiar by some fine striation in addition to the coarse puncturation of gaster tergite 1, a character that is also present in a sample from Sarawak, Borneo (NHMW). A distinct indention of the apex of the petiolar node is present in the workers from Laguna and in the gyne from Palawan, but absent in the Masbate worker.
Chen et al. (2019) - China: The species is similar to Anochetus lanyuensis, Anochetus validus, and Anochetus victoriae, but well separated from them by the following characters: dorsal outline of pronotum gradually sloping anteriorly, not forming a straight outline with mesonotum and propodeum; pronotal disc with dense inverted U-shaped rugae; scapes just reaching to posterior corner of head.
Keys including this Species
- Key to Australian Anochetus Species
- Key to the Anochetus Species of Asia, Melanesia and the Pacific Region
- Key to Anochetus of India
- Key to Micronesian Ants
- Key to Anochetus of the Philippines
- Key to Chinese species of Anochetus
Distribution
Southern India east through SE Asia to Australia and onwards to the Cook Islands.
Within Australia this is one of the most widely distributed and commonly encountered species, occurring from the Kimberleys eastward through the Top End and then throughout eastern Queensland south into north-eastern New South Wales. It is most commonly encountered in rainforest habitats but also extends into dry sclerophyll woodlands. It has only rarely been found outside forested sites. (Shattuck and Slipinska 2012)
Latitudinal Distribution Pattern
Latitudinal Range: 32.9141° to -31.08333015°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Australasian Region: Australia, New Caledonia.
Indo-Australian Region: Borneo, Cook Islands, Fiji, Indonesia (type locality), Kiribati, Krakatau Islands, Malaysia (type locality), Marshall Islands, Micronesia (Federated States of), New Guinea, Northern Mariana Islands, Palau, Philippines (type locality), Samoa (type locality), Singapore, Solomon Islands, Tokelau, Tonga, Wallis and Futuna Islands.
Oriental Region: Bangladesh, Cambodia, India (type locality), Laos, Myanmar (type locality), Nicobar Island, Pakistan, Sri Lanka, Thailand, Vietnam.
Palaearctic Region: China.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
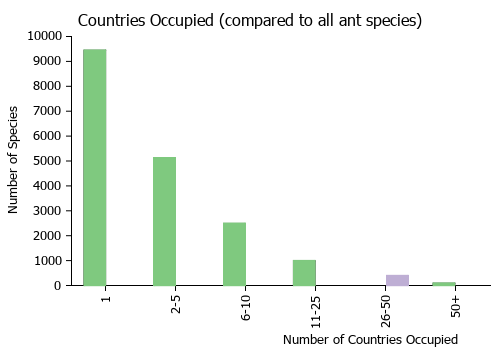
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Elevation Range
| Species | Elevation (m asl) | |||||
|---|---|---|---|---|---|---|
| 200 | 400 | 600 | 800 | 1000 | 1200 | |
| Anochetus graeffei | 0-10 | |||||
| Shading indicates the bands of elevation where species was recorded. Numbers are the percentage of total samples containing this species. | ||||||
Biology
This is a small ant species which is the most common type of Anochetus species found in Srilanka and India. They feed exclusively on small insects such as termites, small earwigs, Scutigerella, etc. They have small colonies with less than 100 workers. They are monogynous but sometimes found having 2 or 3 queens as well.
Castes
Worker
                
| |
| . | |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- graeffei. Anochetus graeffei Mayr, 1870b: 961 (w.) SAMOA (Upolu).
- Type-material: lectotype worker (by designation of Brown, 1978c: 586), 3 paralectotype workers.
- Type-locality: Samoa (“Schiffer Is”): Upolu I. (no collector’s name; probably Gräffe).
- Type-depository: NHMW.
- Mayr, 1876: 86 (q.); Wheeler, G.C. & Wheeler, J. 1971b: 1212 (l.); Imai, et al. 1984: 5 (k.).
- Status as species: Mayr, 1876: 86; Emery, 1884a: 378 (in key); Dalla Torre, 1893: 47; Emery, 1911d: 108; Emery, 1914b: 180; Forel, 1915b: 35; Mann, 1919: 300; Mann, 1921: 426; Wheeler, W.M. 1924b: 243; Santschi, 1928a: 44; Wheeler, W.M. 1935g: 15; Chapman & Capco, 1951: 40; Wilson, 1959a: 507; Wilson & Taylor, 1967: 32; Taylor, 1967b: 1094; Taylor, 1976a: 80; Brown, 1978c: 556, 586; Taylor & Brown, 1985: 20; Taylor, 1987a: 7; Radchenko, 1993a: 77; Dlussky, 1994: 53; Bolton, 1995b: 64; Zhou, 2001b: 30; Wetterer, 2002: 128; Wetterer & Vargo, 2003: 416; Jaitrong & Nabhitabhata, 2005: 12; Clouse, 2007b: 261; Mohanraj, et al. 2010: 6; Zhou & Ran, 2010: 103; Pfeiffer, et al. 2011: 55; Guénard & Dunn, 2012: 58; Sarnat & Economo, 2012: 146; Shattuck & Slipinska, 2012: 11 (redescription); Zettel, 2012: 164; Bharti & Wachkoo, 2013a: 142 (in key); Sarnat, et al. 2013: 73; Ramage, 2014: 151; Jaitrong, Guénard, et al. 2016: 40; Chen, Z., Yang & Zhou, 2019: 52 (redescription); Dias, R.K.S. et al. 2020: 101; Khachonpisitsak, et al. 2020: 138; Wang, W.Y., Soh, et al. 2022: 115.
- Senior synonym of amati: Wilson, 1959a: 507; Brown, 1978c: 557; Bolton, 1995b: 64; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11.
- Senior synonym of minutus: Wilson, 1959a: 507; Brown, 1978c: 557; Bolton, 1995b: 64; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11.
- Senior synonym of oceanicus: Wilson, 1959a: 507; Wilson & Taylor, 1967: 32; Brown, 1978c: 557; Taylor, 1987a: 8; Bolton, 1995b: 64; Shattuck & Slipinska, 2012: 11; Chen, Z., Yang & Zhou, 2019: 53.
- Senior synonym of punctiventris: Wilson, 1959a: 507; Brown, 1978c: 557; Bolton, 1995b: 64; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11; Chen, Z., Yang & Zhou, 2019: 52.
- Senior synonym of rudis: Brown, 1978c: 557, 586; Bolton, 1995b: 64; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11; Chen, Z., Yang & Zhou, 2019: 52.
- Senior synonym of taylori: Brown, 1978c: 557, 586; Bolton, 1995b: 64; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11.
- Distribution
- Austral: Australia, New Caledonia.
- Malesian: Fiji Is, French Polynesia, Indonesia (Aru Is, Flores, Java, Kalimantan, Prinsen, Sulawesi, Sumba), Malaysia (Peninsula, Sabah), Micronesia, Papua New Guinea, Philippines (Luzon, Masbate, Negros, Palawan), Samoa, Singapore, Solomon Is, Timor Leste, Tonga, Vanuatu.
- Oriental: China, India (+ Nicobar Is), Laos, Myanmar, Sri Lanka, Thailand, Vietnam.
- amati. Anochetus amati Karavaiev, 1925c: 285, fig. 8 (q.) INDONESIA (Aru Is).
- Type-material: holotype queen.
- Type-locality: Indonesia: Aru Is, Wammar I., 1912-13, Nr. 2560 (W. Karawaiew).
- Type-depository: SIZK.
- Status as species: Chapman & Capco, 1951: 39.
- Junior synonym of graeffei: Wilson, 1959a: 507; Brown, 1978c: 557; Bolton, 1995b: 63; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11.
- minutus. Anochetus minutus Karavaiev, 1925c: 288, fig. 10 (w.q.) WEST MALAYSIA.
- Type-material: syntype workers (number not stated, “a large number”), 1 syntype queen.
- [Notes (i): Baroni Urbani, 1973b: 143, cites 2w syntypes NHMB; (ii) Kostyuk, 1976: 93, cites 6w syntypes SIZK; (iii) Radchenko, Fisher, et al. 2023: 14, cite 4w syntypes SIZK.]
- Type-locality: Malaysia: Johore (Malacca), Segamat, 1912-13, (John Nr. 395), Nr 2757 (O. John).
- Type-depositories: NHMB, SIZK.
- Status as species: Yasumatsu, 1940b: 313; Chapman & Capco, 1951: 40.
- Junior synonym of graeffei: Wilson, 1959a: 507; Brown, 1978c: 557; Bolton, 1995b: 65; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11.
- oceanicus. Anochetus punctiventris subsp. oceanicus Emery, 1897c: 597 (w.) NEW GUINEA (Papua New Guinea).
- Type-material: syntype workers (number not stated).
- Type-localities: Papua New Guinea (“Nova-Guinea, Colonia Germanica”): Friedrich-Wilhemshafen (= Madang) (L. Biró), Papua New Guinea: Berlinhafen (= Aitepe) (L. Biró).
- Type-depositories: HNHM, MSNG.
- Emery, 1914f: 400 (q.m.).
- Subspecies of punctiventris: Forel, 1901b: 6; Dahl, 1901: 12; Emery, 1911d: 108; Emery, 1914f: 400; Mann, 1919: 300; Wheeler, W.M. & Chapman, 1925: 71; Wheeler, W.M. 1935g: 15; Chapman & Capco, 1951: 41; Baltazar, 1966: 238.
- Junior synonym of graeffei: Wilson, 1959a: 507; Wilson & Taylor, 1967: 32; Brown, 1978c: 557; Taylor, 1987a: 8; Bolton, 1995b: 65; Shattuck & Slipinska, 2012: 11; Chen, Z., Yang & Zhou, 2019: 53.
- punctiventris. Anochetus punctiventris Mayr, 1879: 659 (w.) INDIA (West Bengal).
- Type-material: syntype workers (number not stated).
- Type-localities: India: Calcutta ((G.A.J. Rothney), India: Nuddea District, N of Calcutta (G.A.J. Rothney).
- Type-depositories: MSNG, NHMW.
- Forel, 1900c: 63 (q.).
- Status as species: Emery, 1883: 148; Emery, 1884a: 378 (in key); Dalla Torre, 1893: 48; Emery, 1897c: 597; Forel, 1900c: 63; Rothney, 1903: 96; Bingham, 1903: 41; Emery, 1911d: 109; Viehmeyer, 1916a: 115; Wheeler, W.M. 1924b: 242; Wheeler, W.M. 1927h: 84; Wheeler, W.M. 1937a: 21; Chapman & Capco, 1951: 41; Tiwari, 1999: 20 (error); Mathew & Tiwari, 2000: 291 (error).
- Junior synonym of graeffei: Wilson, 1959a: 507; Brown, 1978c: 557; Bolton, 1995b: 65; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11; Chen, Z., Yang & Zhou, 2019: 52.
- [Note: punctiventris is erroneously reinstated and included in a key to Odontomachus species: Mathew & Tiwari, 2000: 289.]
- rudis. Anochetus rudis Emery, 1889b: 499 (w.) MYANMAR.
- Type-material: syntype workers (number not stated).
- Type-localities: Myanmar (“Birmania”): Mandalay, 1885-87 (L. Fea), Myanmar: Prome (= Pyay), 1885-87 (L. Fea).
- Type-depository: MSNG.
- Subspecies of punctiventris: Forel, 1900c: 63; Emery, 1911d: 109; Chapman & Capco, 1951: 41.
- Status as species: Dalla Torre, 1893: 48; Emery, 1895k: 463; Bingham, 1903: 41.
- Junior synonym of graeffei: Brown, 1978c: 577, 586; Bolton, 1995b: 65; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11; Chen, Z., Yang & Zhou, 2019: 52.
- taylori. Anochetus punctiventris r. taylori Forel, 1900c: 63 (w.) INDIA (Tamil Nadu, Karnataka, Maharashtra).
- Type-material: syntype workers (number not stated).
- [Note: Baroni Urbani, 1973b: 143, cites 1w syntype NHMB.]
- Type-localities: India: Coonoor (R.C. Wroughton), India: Belgaum (R.C. Wroughton), India: Poona (R.C. Wroughton).
- Type-depositories: MHNG, NHMB.
- Status as species: Bingham, 1903: 43; Wheeler, W.M. 1937a: 22.
- Subspecies of punctiventris: Emery, 1900d: 671; Emery, 1911d: 108; Forel, 1911i: 215; Wheeler, W.M. 1924b: 242; Chapman & Capco, 1951: 41; Tiwari, 1999: 20 (error).
- Junior synonym of graeffei: Brown, 1978c: 557, 586; Bolton, 1995b: 66; Zhou, 2001b: 30; Shattuck & Slipinska, 2012: 11.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Type Material
- Anochetus graeffei: Syntype, workers (4 examined by Brown, 1978), Upolu Island, Samoa, Naturhistorisches Museum Wien, Vienna.
- Anochetus amati: Holotype, queen, Aru Island, Indonesia.
- Anochetus minutus: Syntype, worker(s) and queen(s), Segamat, Johore, Malaysia, Naturhistorisches Museum, Basel.
- Anochetus punctiventris subsp. oceanicus: Syntype, workers, Madang (as Friedrich-Wilhelmshafen) and Aitape (as Berlinhafen), Papua New Guinea.
- Anochetus punctiventris: Syntype, workers, Calcutta and the "Nuddea District", India.
- Anochetus rudis: Syntype, Myanmar.
- Anochetus ruginotus: Holotype, worker, Luzon, Philippines, Berlin Museum für Naturkunde der Humboldt-Universität.
- Anochetus punctiventris r. taylori: Syntype, syntype, Coonoor, Madras State, India.
Taxonomic Notes
Shattuck and Slipinska (2012) - This is one of the most widely distributed species within the genus, occurring from southern India east through SE Asia to Australia and onwards to the Cook Islands; it is also one of the most morphologically variable (Brown, 1978). The concept of this species adopted by Shattuck & Slipinska (2012) follows that proposed by Brown (1978). While a detailed study of the entire species, including examination of specimens from throughout its broad range, was outside the scope of their study, a preliminary analysis does not suggest obvious subdivisions and Brown's interpretation of the variation he noted as intra- rather than interspecific is accepted. Additionally, an examination of specimens from inland north Queensland which Brown flagged as possibly belonging to a separate but closely related species could not be confirmed and material from this region is treated as belong to A. graeffei.
Zettel (2012) - The taxonomy and synonymy of A. graeffei was treated by WILSON (1959) and BROWN (1978). Following these authors, A. graeffei is very widely distributed from India to Australia and a most polymorphic species. According to BROWN (1978) this decision is not final: “The bounds of graeffei variation, and whether or not the species divides into sibling species, are ripe subjects for future gammataxonomic studies.” Variation concerns size, colour, and sculpture (most notably on head, pronotum and gaster tergite 1).
A hitherto undescribed, but important peculiarity of both A. graeffei s.l. and A. ruginotus are short setae on the compound eyes; it is undescribed whether or not this character is also present in other species of Brown's A. graeffei group. Less obvious, shorter setae have been also observed in a few species of the A. risii group.
Description
Shattuck and Slipinska (2012) - Worker description. Body smaller (head length < 1.14mm), with abundant erect or semierect hairs. Eyes very small (eye length < 0.16mm). Sculpturing on front of head nearly reaching posterior margin and extending slightly laterally. Dorsum of head with abundant semierect hairs as well as a few erect hairs. Scapes not reaching posterolateral corners ('lobes') of head; with abundant, slightly elevated pubescence and a limited number of erect hairs. Pronotum with characteristic punctate, irregularly rugose sculpture. Anterior section of pronotum with transverse wrinkles and ridges. Mesonotum and dorsum of propodeum with coarse striate-rugose sculpture. Dorsum of propodeum rounded laterally, with slightly rounded angle and numerous erect hairs. Metapleuron smooth and shining anteriorly. In anterior view petiolar node with apex rounded. Erect hairs on hind tibiae short and scattered. Colour from yellowbrown to brown, head from yellow to yellow-brown, antennae, mandibles and legs yellow or yellow-brown.
Measurements. Worker (n = 5): CI 92–93; EI 14–15; EL 0.13–0.15; HL 0.95–1.13; HW 0.89–1.06; HFL 0.77–0.89; ML 1.09–1.30; MandL 0.52–0.59; MTL 0.54–0.66; PronI 59–63; PronW 0.52–0.64; SL 0.76–0.87; SI 83–89.
Karyotype
- See additional details at the Ant Chromosome Database.
 Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Karyotype data or Search these data. See also a list of all data tables or learn how data is managed.
- n = 15; 19, 2n = 30; 38 (India; Indonesia) (Imai et al., 1984; Imai et al., 1985; Mariano et al., 2015).
References
- Aguiar, H.J.A.C., Barros, L.A.C., Silveira, L.I., Petitclerc, F., Etienne, S., Orivel, J. 2020. Cytogenetic data for sixteen ant species from North-eastern Amazonia with phylogenetic insights into three subfamilies. Comparative Cytogenetics 14(1): 43–60 (doi:10.3897/CompCytogen.v14i1.46692).
- Brown, W. L., Jr. 1978c. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section B. Genus Anochetus and bibliography. Stud. Entomol. 20: 549-638. (page 557, senior synonym of rudis, ruginotus and taylori)
- Burwell, C.J., Nakamura, A. 2020. Rainforest ants (Hymenoptera: Formicidae) along an elevational gradient at Eungella in the Clarke Range, Central Queensland coast, Australia. Proceedings of the Royal Society of Queensland 125: 43-63.
- Chen, Z., Yang, Z., Zhou, S. 2019. Review of the ant genus Anochetus Mayr, 1861 (Hymenoptera, Formicidae) from China, with revival of the valid status of Anochetus gracilis. Journal of Hymenoptera Research 68: 49–74 (DOI 10.3897/jhr.68.30784).
- Dias, R.K.S., Kosgamage, K.R.K.A. 2013. Occurrence and species diversity of ground-dwelling worker ants (Family: Formicidae) in selected lands in the dry zone of Sri Lanka. Journal of Science of the University of Kelaniya Sri Lanka 7: 55-72 (doi:10.4038/josuk.v7i0.6233).
- Dias, R.K.S., Rajapaksa, R.P.K.C. 2017. Geographic records of subfamilies, genera and species of ants (Hymenoptera: Formicidae) in the four climatic zones of Sri Lanka: A review. Journal of Science of the University of Kelaniya Sri Lanka 11, 23-45. (doi:10.4038/josuk.v11i2.7999).
- Emery, C. 1897c. Formicidarum species novae vel minus cognitae in collectione Musaei Nationalis Hungarici quas in Nova-Guinea, colonia germanica, collegit L. Biró. Természetr. Füz. 20: 571-599.
- General, D.E.M. 2021. A preliminary checklist of the ants (Hymenoptera: Formicidae) of the Mt. Pantaron Range, Bukidnon Province, Mindanao Island, Philippines. Halteres, 12:4-14 (doi:10.5281/ZENODO.5371745).
- General, D.E.M., Buenavente, P.A.C., Rodriguez, L.J.V. 2020. A preliminary survey of nocturnal ants, with novel modifications for collecting nocturnal arboreal ants. Halteres 11: 1-12 (doi:10.5281/ZENODO.3707151).
- Hasin, S., Tasen, W. 2020. Ant community composition in urban areas of Bangkok, Thailand. Agriculture and Natural Resources 54: 507-514 (doi:10.34044/j.anres.2020.54.5.07).
- Hasin, S., Tasen, W., Ohashi, M., Boonriam, W., Yamada, A. 2021. Yellow crazy ants (Anoplolepis gracilipes [Smith, F., 1857]: Hymenoptera: Formicidae) threaten community of ground-dwelling arthropods in dry evergreen forests of Thailand. Agriculture and Natural Resources 55: 634-643 (doi:10.34044/j.anres.2021.55.4.14).
- Heterick, B.E. 2021. A guide to the ants of Western Australia. Part I: Systematics. Records of the Western Australian Museum, Supplement 86, 1-245 (doi:10.18195/issn.0313-122x.86.2021.001-245).
- Heterick, B.E. 2022. A guide to the ants of Western Australia. Part II: Distribution and biology. Records of the Western Australian Museum, supplement 86: 247-510 (doi:10.18195/issn.0313-122x.86.2022.247-510).
- Imai, H. T.; Baroni Urbani, C.; Kubota, M.; Sharma, G. P.; Narasimhanna, M. H.; Das, B. C.; 1984. Karyological survey of Indian ants. Jpn. J. Genet. 59: 1-32 (page 5, karyotype described)
- Khachonpisitsak, S., Yamane, S., Sriwichai, P., Jaitrong, W. 2020. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). ZooKeys 998, 1–182 (doi:10.3897/zookeys.998.54902).
- Lakho, G.M., Khatri, I., Rustamani, M.A. 2020. First record of trap-jaw ant species of genus Anochetus Mayr, 1861 (Hymenoptera: Formicidae) from Pakistan. Sindh University Research Journal (Science Series) 52(4), 301-304 (doi:10.26692/sujo/2020.12.45).
- Latumahina, F., Borovanska, M., Musyafa, Sumardi, Susetya Putra, N., Janda, M. 2015. Ants of Ambon Island – diversity survey and checklist. ZooKeys 472, 43–57 (doi:10.3897/zookeys.472.8441).
- Liu, C., Fischer, G., Hita Garcia, F., Yamane, S., Liu, Q., Peng, Y.Q., Economo, E.P., Guénard, B., Pierce, N.E. 2020. Ants of the Hengduan Mountains: a new altitudinal survey and updated checklist for Yunnan Province highlight an understudied insect biodiversity hotspot. ZooKeys 978, 1–171 (doi:10.3897/zookeys.978.55767).
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Mayr, G. 1870b. Neue Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 20: 939-996 (page 961, worker described)
- Mayr, G. 1876. Die australischen Formiciden. J. Mus. Godeffroy 12: 56-115 (page 86, queen described)
- Radchenko, A.G., Fisher, B.L., Esteves, F.A., Martynova, E.V., Bazhenova, T.N., Lasarenko, S.N. 2023. Ant type specimens (Hymenoptera, Formicidae) in the collection of Volodymyr Opanasovych Karawajew. Communication 1. Dorylinae, Poneromorpha and Pseudomyrmecinae. Zootaxa, 5244(1), 1–32 (doi:10.11646/zootaxa.5244.1.1).
- Shattuck, S.O. & Slipinska, E. 2012. Revision of the Australian species of the ant genus Anochetus (Hymenoptera Formicidae). Zootaxa 3426, 1–28.
- Wang, W.Y., Soh, E.J.Y., Yong, G.W.J., Wong, M.K.L., Benoit Guénard, Economo, E.P., Yamane, S. 2022. Remarkable diversity in a little red dot: a comprehensive checklist of known ant species in Singapore (Hymenoptera: Formicidae) with notes on ecology and taxonomy. Asian Myrmecology 15: e015006 (doi:10.20362/am.015006).
- Wheeler, G. C.; Wheeler, J. 1971b. Ant larvae of the subfamily Ponerinae: second supplement. Ann. Entomol. Soc. Am. 6 64: 1197-1217 (page 1212, larva described)
- Wilson, E. O. 1959c. Studies on the ant fauna of Melanesia V. The tribe Odontomachini. Bulletin of the Museum of Comparative Zoology 120: 483-510 (page 507, senior synonym of amati, minutus, oceanicus and punctiventris)
- Yamane, S., Tanaka, H.O., Hasimoto, Y., Ohashi, M., Meleng, P., Itioka, T. 2021. A list of ants from Lambir Hills National Park and its vicinity, with their biological information: Part II. Subfamilies Leptanillinae, Proceratiinae, Amblyoponinae, Ponerinae, Dorylinae, Dolichoderinae, Ectatomminae and Formicinae. Contributions from the Biological Laboratory, Kyoto University 31, 87–157.
- Zettel, H. 2012. New trap-jaw ant species of Anochetus Mayr, 1861 (Hymenoptera: Formicidae) from the Philippine Islands, a key and notes on other species. Myrmecological News. 16:157-167.
References based on Global Ant Biodiversity Informatics
- Abe T., S. Yamane, and K. Onoyama. Ants collected on the Krakatau Islands 100 years after the great eruptions. Biogeography 14: 65-75.
- Basu P., N. Tak, and A. K. Sanyal. 2013. Ants (insecta: Hymenoptera: Formicidae) of Bethuadahari wildlife sanctuary, Nadia, West Bengal, India. Rec. zool, Surv. India: 113(4): 17-22.
- Bharti H., Y. P. Sharma, M. Bharti, and M. Pfeiffer. 2013. Ant species richness, endemicity and functional groups, along an elevational gradient in the Himalayas. Asian Myrmecology 5: 79-101.
- Brown Jr., W.L. 1978. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, Tribe Ponerini, Subtribe Odontomachiti, Section B. Genus Anochetus and Bibliography. Studia Entomologia 20(1-4): 549-XXX
- Brown W.L. Jr. 1978. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section B. Genus Anochetus and bibliography. Studia Ent. 20(1-4): 549-638.
- CSIRO Collection
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Chazeau J., H. Jourdan, L. Bonnet de Larbogne, J. Konghouleux, and T. Potiaroa. 2003. Etude floristique et faunistique de la foret seche de Nekoro, 2 eme partie: evaluation de l'integrite de la faune par l'etude de la myrmecofaune. Lettre de Commande Programme Forêt Sèche /lRD N°l 54/2002/CP
- Chen Y. Q., Q. Li, Y. L. Chen, Z. X. Lu, X. Y. Zhou. 2011. Ant diversity and bio-indicators in land management of lac insect agroecosystem in Southwestern China. Biodivers. Conserv. 20: 3017-3038.
- Chen Z., Z. Yang, and S. Zhou. 2019. Review of the ant genus Anochetus Mayr, 1861 (Hymenoptera, Formicidae) from China, with revival of the valid status of Anochetus gracilis. Journal of Hymenoptera Research 68: 49–74.
- Clouse R. M. 2007. The ants of Micronesia (Hymenoptera: Formicidae). Micronesica. 39: 171-295.
- Clouse, R.M. 2007. The ants of Micronesia (Hymenoptera: Formicidae), Micronesica 39(2): 171-295.
- Dad J. M., S. A. Akbar, H. Bharti, and A. A. Wachkoo. 2019. Community structure and ant species diversity across select sites ofWestern Ghats, India. Acta Ecologica Sinica 39: 219–228.
- Dias R. K. S. 2002. Current knowledge on ants of Sri Lanka. ANeT Newsletter 4: 17- 21.
- Dias R. K. S. 2013. Diversity and importance of soil-dweeling ants. Proceedings of the National Symposium on Soil Biodiversity, chapt 4, pp 19-22.
- Dias R. K. S., K. R. K. A. Kosgamage, and H. A. W. S. Peiris. 2012. The Taxonomy and Conservation Status of Ants (Order: Hymenoptera, Family: Formicidae) in Sri Lanka. In: The National Red List 2012 of Sri Lanka; Conservation Status of the Fauna and Flora. Weerakoon, D.K. & S. Wijesundara Eds., Ministry of Environment, Colombo, Sri Lanka. p11-19.
- Dias R. K. S., and H. A. W. S. Peiris. 2015. Ground-dwelling ant assemblages (Family: Formicidae) in six coconut (Cocos nucifera L. 1753) plantations in Sri Lanka. Journal ofInsect Biodiversity 3(14): 1–10. http://dx.doi.org/10.12976/jib/2015.3.14
- Dias R. K. S., and K. R. K. Anuradha Kosgamage. 2012. Occurrence and species diversity of ground-dwelling worker ants (Family: Formicidae) in selected lands in the dry zone of Sri Lanka. J. Sci. Univ. Kelaniya 7: 55-72.
- Dias R. K. S., and R. P. K. C. Rajapaksa. 2016. Geographic records of subfamilies, genera and species of ants (Hymenoptera: Formicidae) in the four climatic zones of Sri Lanka: a review. J. Sci. Univ. Kelaniya 11(2): 23-45.
- Dlussky G.M. 1994. Zoogeography of southwestern Oceania. Zhivotnoe naselenie ostrovov Iugo-Zapadnoi Okeanii ekologo-geograficheskie issledovanii 48-93.
- Emery C. 1883. Alcune formiche della Nuova Caledonia. Bullettino della Società Entomologica Italiana 15: 145-151.
- Emery C. 1897. Formicidarum species novae vel minus cognitae in collectione Musaei Nationalis Hungarici quas in Nova-Guinea, colonia germanica, collegit L. Biró. Természetrajzi Füzetek 20: 571-599.
- Emery C. 1911. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125.
- Emery C. 1914. Formiche d'Australia e di Samoa raccolte dal Prof. Silvestri nel 1913. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici 8: 179-186.
- Emery C. Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei. Annali del Museo Civico di Storia Naturale 40: 661-722.
- Emery, C. 1914. Les fourmis de la Nouvelle-Calédonie et des îles Loyalty. Nova Caledonia. A. Zoologie 1:393-437.
- Emery, C. "Formiche raccolte da Elio Modigliani in Sumatra, Engano e Mentawei." Annali del Museo Civico di Storia Naturale Giacomo Doria (Genova) (2) 20, no. 40 (1900): 661-722.
- Field Museum Collection, Chicago, Illinois (C. Moreau)
- Forel A. 1900. Les Formicides de l'Empire des Indes et de Ceylan. Part VI. J. Bombay Nat. Hist. Soc. 13: 52-65.
- Forel A. 1901. Formiciden aus dem Bismarck-Archipel, auf Grundlage des von Prof. Dr. F. Dahl gesammelten Materials. Mitt. Zool. Mus. Berl. 2: 4-37.
- Forel A. 1911. Ameisen aus Ceylon, gesammelt von Prof. K. Escherich (einige von Prof. E. Bugnion). Pp. 215-228 in: Escherich, K. Termitenleben auf Ceylon. Jena: Gustav Fischer, xxxii + 262 pp.
- Greenslade P.J.M. and Greenslade Penelope. 1977. Some Effects of Vegetation Cover and Disturbance on a Tropical Ant Fauna. Insectes Sociaux 24(2): 163-182
- Greenslade P.J.M. and P. Greenslade. 1977. Some effects of vegetation cover and disturbance on a tropical ant fauna. Insectes Sociaux 24(2): 163-182.
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Hashimoto Y., and M. Mohamed. 2011. Ground-dwelling ant diversity in Maliau Basin, Borneo: evaluation of hand-sorting methods to estimate ant diversity. Tropics 19(2): 85-92.
- Herwina H., and K. Nakamura. 2007. Ant species diversity study using pitfall traps in a small yard in Bogor Botanic garden, West Java, Indonesia. Treubia 35: 99-116.
- Hua Li-zhong. 2006. List of Chinese insects Vol. IV. Pages 262-273. Sun Yat-sen university Press, Guangzhou. 539 pages.
- Imai H. T., C. Baroni Urbani, M. Kubota, G. P. Sharma, M. H. Narasimhanna, B. C. Das, A. K. Sharma, A. Sharma, G. B. Deodikar, V. G. Vaidya, and M. R. Rajasekarasetty. 1984. Karyological survey of Indian ants. Japanese Journal of Genetics 59: 1-32.
- Imai H. T., M. Kubota, W. L. Brown, Jr., M. Ihara, M. Tohari, and R. I. Pranata. 1985. Chromosome observations on tropical ants from Indonesia. Annu. Rep. Natl. Inst. Genet. Jpn. 35: 46-48.
- Ito, F.; Yamane, S.; Eguchi, K.; Noerdjito, W. A.; Kahono, S.; Tsuji, K.; Ohkawara, K.; Yamauchi, K.; Nishida, T.; Nakamura, K. 2001. Ant species diversity in the Bogor Botanic Garden, West Java, Indonesia, with descriptions of two new species of the genus Leptanilla (Hymenoptera, Formicidae). Tropics 10:379-404.
- Jaitrong W., B. Guenard, E. P. Economo, N. Buddhakala, and S. Yamane. 2016. A checklist of known ant species of Laos (Hymenoptera: Formicidae). Asian Myrmecology 8: 1-32. DOI: 10.20362/am.008019
- Jaitrong W.; Nabhitabhata, J. 2005. A list of known ant species of Thailand. The Thailand Natural History Museum Journal 1(1): 9-54.
- Janda M., G. D. Alpert, M. L. Borowiec, E. P. Economo, P. Klimes, E. Sarnat, and S. O. Shattuck. 2011. Cheklist of ants described and recorded from New Guinea and associated islands. Available on http://www.newguineants.org/. Accessed on 24th Feb. 2011.
- Jennings J. T., L. Krogmann, and C. Burwell. 2013. Review of the hymenopteran fauna of New Caledonia with a checklist of species. Zootaxa 3736(1): 1-53.
- Jourdan H., J. Konghouleux. 2005. Bilan entomologique des noyaux forestiers dits, S2 à S5, à Prony, définies par Goro Nickel SA. Rapport d'Expertise IRD / Goro Nickel SA, 15 pages.
- Kami K.S., and S. E. Miller. 1998. Samoan insects and related arthropods: checklist and bibliography. Bishop Museum Technical Report 13, pp 121.
- Kami KS & Miller SE. 1998. Samoan insects and related arthropods: checklist and bibliography. Bishop Museum Technical Report No. 13.
- Karavaiev V. 1925. Ponerinen (Fam. Formicidae) aus dem Indo-Australischen Gebiet. (Schluss). Konowia 4: 276-296.
- Karavaiev V. 1926. Ameisen aus dem Indo-Australischen Gebiet. Treubia 8: 413-445.
- Khoo Y.H. 1990. A note on the Formicidae (Hymenoptera) from pitfall traps at Ulu Kinchin, Pahang, Malaysia. Malayan Nature Journal 43: 290-293.
- Latumahina F., M. Borovanska, N. S. Putra, and M. Janda. 2015. Ants of Ambon Island diversity survey and checklist. ZooKeys 472: 4357.
- Li Z.h. 2006. List of Chinese Insects. Volume 4. Sun Yat-sen University Press
- Lopwichan S., and S. Khachonpisitsak. 2015. Ant diversity in Nong Tha Yu Arboretum, Si Racha District, Chon Buri Province. Proceedings The 7 th National Science Research Conference. 30-31 March 2015. Naresuan University.
- Lucky A., L. E. Alonso, E. Sarnat, and J. Hulr. 2015. Ants and scolytine beetles. In: Richards, S.J. and N. Whitmore (editors) 2015. A rapid biodiversity assessment of Papua New Guinea's Hindenburg Wall region. Wildlife Conservation Society Papua New Guinea Program. Goroka, PNG.
- Mann W. M. 1919. The ants of the British Solomon Islands. Bulletin of the Museum of Comparative Zoology 63:273-391.
- Mann W. M. 1921. The ants of the Fiji Islands. Bulletin of the Museum of Comparative Zoology 64: 401-499.
- Mann William. 1916. The Ants of the British Solomon Islands. Bulletin of the Museum of Comparative Zoology at Harvard College 63(7): 273-391
- Mann, W.M. 1919. The ants of the British Solomon Islands. Bulletin of the Museum of Comparative Zoology of Harvard College 63: 273-391
- Mohanraj P., M. Ali, and K. Veerakumari. 2010. Formicidae of the Andaman and Nicobar Islands (Indian Ocean: Bay of Bengal). Journal of Insect Science 10: Article 172
- Mohanraj, P., M. Ali and K. Veenakumari. 2010. Formicidae of the Andaman and Nicobar Islands (Indian Ocean: Bay Of Bengal). Journal of Insect Science 10:172.
- Musthak Ali T. M. 1982. Ant fauna (Hymenoptera: Formicidae) of Bangalore with observations on their nesting and foraging habits. Thesis Abstracts. Haryana Agricultural University 8: 370-371.
- Pfeiffer M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E. Y.; Kohout, R. J. 2011. The Formicidae of Borneo (Insecta: Hymenoptera): a preliminary species list. Asian Myrmecology 4:9-58
- Philpott S.M., P. Bichier, R.A. Rice, and R. Greenberg. 2008. Biodiversity conservation, yield, and alternative products in coffee agroecosystems in Sumatra, Indonesia. Biodivers. Conserv. 17: 1805-1820. Data obtained from Stacy Philpott
- Radchenko A. G. 1993. Ants from Vietnam in the collection of the Institute of Zoology, PAS, Warsaw. I. Pseudomyrmicinae, Dorylinae, Ponerinae. Annales Zoologici (Warsaw) 44: 75-82.
- Rajan P. D., M. Zacharias, and T. M. Mustak Ali. 2006. Insecta: Hymenoptera: Formicidae. Fauna of Biligiri Rangaswamy Temple Wildlife Sanctuary (Karnataka). Conservation Area Series, Zool. Surv. India.i-iv,27: 153-188.
- Rizali A., A. Rahim, B. Sahari, L.B. Prasetyo, and D. Buchori. 2011. Impact of invasive ant species in shaping ant community structure on small islands in Indonesia. Jurnal Biologi Indonesia 7(2): 221-230.
- Rizali A., Clough Y., Buchori D. and Tscharntke T. 2013. Dissimilarity of ant Communities Increases with Precipitation, but not Reduced Land-Use Intensity, in Indonesian Cacao Agroforestry. Diversity. 5: 26-38
- Rizali A., D. J. Lohman, D. Buchori, L. Budi Prasetyo, H. Triwidodo, M. M. Bos, S. Yamane, and C. H. Schulze. 2009. Ant communities on small tropical islands: effects of island size and isolation are obscured by habitat disturbance and tramp ant species. Journal of Biogeography 37(2): 229-236.
- Rizali A., M. M. Bos, D. Buchori, Sk. Yamane, and C. H. Schulze. 2008. Ants in tropical urban habitats: the myrmecofauna in a densely populated area of Bogor, West Java, Indonesia. Hayati Journal of Biosciences 15(2): 77-84.
- Rizali A., M.M. Bos, D. Buchori, Sk. Yamane, C. Hans, and J. Schulze. 2008. Ants in tropical urban habitats: the myrmecofauna in a densely populated area of Bogor, West Java, Indonesia. Hayati Journal of Biosciences 77-84.
- Room P. M. 1975. Diversity and organization of the ground foraging ant faunas of forest, grassland and tree crops in Papua Nez Guinea. Aust. J. Zool. 23: 71-89.
- Room, P.M. 1975. Relative Distributions of Ant Species in Cocoa Plantations in Papua New Guinea Relative Distributions of Ant Species in Cocoa Plantations in Papua New Guinea. Journal of Applied Ecology 12(1):47-61
- Santschi F. 1928. Formicidae (Fourmis). Insects Samoa. 5: 41-58.
- Sarnat Eli M. 2009. The Ants [Hymenoptera: Formicdiae] of Fiji: Systematics, Biogeography and Conservation of an Island Arc Fauna. 80-252
- Shattuck S. O., and E. Slipinska. 2012. Revision of the Australian species of the ant genus Anochetus (Hymeoptera: Formicidae). Zootaxa 3426: 1-28.
- Sheela S. 2008. Handbook of Hymenoptera, Formicidae. Zoological Survey of India, 56 pages
- Song Y., Z Xu, C Li, N. Zhang, L. Zhang, H. Jiang, and F Mo. 2013. An analysis on the ant fauna of the Nangun River Nature Reserve in Yunnan, China. Forest Research 26(6): 773-780.
- Song Y., Z. Xu, C. Li, N. Zhang, L. Zhang, H. Jiang, and F. Mo. 2013. An Analysis on the Ant Fauna of the Nangun river Nature Reserve in Yunnan, China. Forest Research 26(6): 773-780.
- Tak N. 1995. Studies on ants (Formicidae) of Rajasthan - 1 Jodhpur. Hexapoda 7(1): 17-28.
- Tak N. 2008. Ants of Rajasthan. Conserving Biodiversity of Rajasthan Zool. Surv. India. 149-155.
- Tak N. 2009. Ants Formicidae of Rajasthan. Records of the Zoological Survey of India, Occasional Paper No. 288, iv, 46 p
- Tak N. 2010. Insecta: Hymenoptera: Formicidae. Zool. Surv. India, Fauna of Ranthambore National Park, Conservation Area Series 43: 133-144.
- Tak N., and N. S. Rathore. 1996. Ant (Formicidae) fauna of the Thar Desert. Pp. 271-276 in: Ghosh, A. K.; Baqri, Q. H.; Prakash, I. (eds.) 1996. Faunal diversity in the Thar Desert: gaps in research. Jodhpur: Scientific Publishers, xi + 410 pp.
- Tak N., and N. S. Rathore. 2004. Insecta: Hymenoptera: Formicidae. State Fauna Series 8: Fauna of Gujarat. Zool. Surv. India. Pp. 161-183.
- Tak, N. 2009. Ants (Hymenoptera: Formicidae) of the Thar Desert of Rajasthan and Gujarat. in C. Sivaperuman et al. (eds.), Faunal Ecology and Conservation of the Great Indian Desert
- Taylor R. W. 1976. The ants of Rennell and Bellona Islands. Natural History of Rennell Island, British Solomon Islands 7: 73-90.
- Taylor R. W. 1987. A checklist of the ants of Australia, New Caledonia and New Zealand (Hymenoptera: Formicidae). CSIRO (Commonwealth Scientific and Industrial Research Organization) Division of Entomology Report 41: 1-92.
- Terayama M., and S. Haruhiko. 2005. Ants from Guam Island, Mariana islands, Micronesia. Ari 27: 1-5.
- Terayama. M. 2004. Geological and ecological distribution of Japanese ants communities. (translated from Japanese) Reports of the Saitama Prefecture Animal Research Association. 48:27
- Terayama. M. 2004. Geological and ecological distribution of Japanese ants communities. (translated from Japanese) Reports of the Saitama Prefecture Animal Research Association. 48:28
- Tiwari R. N. 1999. Taxonomic studies on ants of southern India (Insecta: Hymenoptera: Formicidae). Memoirs of the Zoological Survey of India 18(4): 1-96.
- Tiwari R. N., B. G. Kundu, S. Roy Chowdhury, and S. N. Ghosh. 2003. Insecta: Hymenoptera: Formicidae. Fauna of Sikkim. Part 4. State Fauna Series. 9.Zool.Surv.India. i-iii, 1-512. Chapter pagination: 467-506.
- Tiwari R.N., B.G. Kundu, S. Roychowdhury, S.N. Ghosh. 1999. Insecta: Hymenoptera: Formicidae. Pp. 211-294 in: Director; Zoological Survey of India (ed.) 1999. Fauna of West Bengal. Part 8. Insecta (Trichoptera, Thysanoptera, Neuroptera, Hymenoptera and Anoplura). Calcutta: Zoological Survey of India, iv + 442 pp.
- Varghese T. 2004. Taxonomic studies on ant genera of the Indian Institute of Science campus with notes on their nesting habits. Pp. 485-502 in : Rajmohana, K.; Sudheer, K.; Girish Kumar, P.; Santhosh, S. (eds.) 2004. Perspectives on biosystematics and biodiversity. Prof. T.C. Narendran commemoration volume. Kerala: Systematic Entomology Research Scholars Association, xxii + 666 pp.
- Viehmeyer H. 1912. Ameisen aus Deutsch Neuguinea gesammelt von Dr. O. Schlaginhaufen. Nebst einem Verzeichnisse der papuanischen Arten. Abhandlungen und Berichte des Königlichen Zoologischen und Anthropologische-Ethnographischen Museums zu Dresden 14: 1-26.
- Ward, Darren F. and James K. Wetterer. 2006. Checklist of the Ants of Fiji. Fiji Arthropods III 85: 23-47.
- Ward, Darren and Beggs, Jacqueline. 2007. Coexistence, habitat patterns and the assembly of ant communities in the Yasawa islands, Fiji. Ant Oecologica. 32:215-223.
- Watanasit S., S. Sonthichai, and N. Noon-anant. 2003. Preliminary survey of ants at Tarutao National Park, Southern Thailand. Songklanakarin J. Sci. Technol. 25(1) : 115-122
- Wetterer, James K. 2002. Ants of Tonga. Pacific Science. 56.2: 125-135.
- Wetterer, James K. and Vargo, Donald Vargo L. 2003. Ants (Hymenoptera: Formicidae) of Samoa. Pacific Science. 57(4):409-419.
- Wheeler W. M. 1937. Additions to the ant-fauna of Krakatau and Verlaten Island. Treubia 16: 21-24.
- Wheeler W.M. 1935. Check list of the ants of Oceania. Occasional Papers of the Bernice Pauahi Bishop Museum 11(11):1-56.
- Wheeler WM. 1935. Checklist of the ants of Oceania. Bernice P Bishop Museum Occasional Papers. 11.11 pg 3-56
- Wheeler, W. M. 1927. The ants of Lord Howe Island and Norfolk Island. Proc. Am. Acad. Arts Sci. 62: 121-153
- Wheeler, William Morton. 1924. Ants of Krakatau and Other Islands in the Sunda Strait. Treubia. 5(1-3):1-20.
- Wheeler, William Morton. 1927. The Ants of Lord Howe Island and Norfolk Island. Proceedings of the American Academy of Arts and Sciences 62(4): 121-153
- Wheeler, William Morton.1935.Checklist of the Ants of Oceania.Occasional Papers 11(11): 3-56
- Widodo E.S., M. Mohamed, and Y. Hashimoto. 2001. Canopy ant diversity assessment in the fragmented rainforest of Sabah, East Malaysia. Nature and Human activities 6: 13-23.
- Wilson E. O. 1959. Studies on the ant fauna of Melanesia V. The tribe Odontomachini. Bulletin of the Museum of Comparative Zoology 120: 483-510.
- Wilson E. O.; Taylor, R. W. 1967. The ants of Polynesia (Hymenoptera: Formicidae). Pacific Insects Monograph 14:1-109.
- Wilson E.O. 1959. Adaptive shift and dispersal in a tropical ant fauna. Evolution 13(1): 122-144.
- Wilson E.O., and G.L. Hunt. 1967. Ant fauna of Futuna and Wallis islands, stepping stones to Polynesia. Pacific Insects 9(4): 563-584.
- Wilson EO, Hunt GL. 1967. Ant fauna of Futuna and Wallis Islands, stepping stones to Polynesia. Pacific Insects 9.4: 563-584.
- Wilson EO, Taylor RW. 1967. The ants of Polynesia. Pacific Insects Monograph 14:1-109.
- Wilson Edward O. 1959. Adaptive Shift and Dispersal in a Tropical Ant Fauna. Evolution 13(1): 122-144
- Wilson, Edward O. and George L. Hunt. 1967. Ant Fauna of Futuna and Wallis Islands, Stepping Stones To Polynesia. Pacific Insects. 9(4):563-584.
- Wilson, Edward O. and Hunt, George L. Jr. 1967. Ant Fauna of Futuna and Wallis Islands, Stepping Stones to Polynesia. Pacific Insects. 9(4):563-584
- Woodcock P., D. P. Edwards, R. J. Newton, C. Vun Khen, S. H. Bottrell, and K. C. Hamer. 2013. Impacts of Intensive Logging on the Trophic Organisation of Ant Communities in a Biodiversity Hotspot. PLoS ONE 8(4): e60756. doi:10.1371/journal.pone.0060756
- Woodcock P., D. P. Edwards, T. M. Fayle, R. J. Newton, C. Vun Khen, S. H. Bottrell, and K. C. Hamer. 2011. The conservation value of South East Asia's highly degraded forests: evidence from leaf-litter ants. Phil. Trans. R. Soc. B. 366: 3256-3264.
- Xu Z. 1999. [An analysis on the ant fauna of the tropical rain forest in Xishuangbanna of China.] Zoological Research 20: 379-384.
- Yamane S. 2013. A Review of the ant fauna of the Krakatau Islands, Indonesia. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. Ser: A, 11: 1-66
- Yasumatsu K. 1940. Beiträge zur Kenntnis der Ameisenfauna Mikronesiens. I. Die Ameisengattung Anochetus Mayr der Karolinen. Annotationes Zoologicae Japonenses 19:312-315.
- Zettel H. 2012. New trap-jaw ant species of Anochetus Mayr, 1861 (Hymenopter: Formicidae) from the Philippine Islands, a key and notes on other species. Myrmecological News 16: 157-167.
- Zhang N. N., Y. Q. Chen, Z. X. Lu, W. Zhang, and K. L. Li. 2013. Species diversity, community structure difference and indicator species of leaf-litter ants in rubber plantations and secondary natural forests in Yunnan, southwestern China. Acta Entomologica Sinica 56(11): 1314-1323.
- Zhang Xiang, and Hou You-Ming. 2009. Five new record genus and thirty one new records species of ants (Hymenoptera; Formicidae) in Fujian Province. Journal of Fujian Agriculture and Forestry University 38(5): 479-484.
- Zryanin V. A. 2011. An eco-faunistic review of ants (Hymenoptera: Formicidae). In: Structure and functions of soil communities of a monsoon tropical forest (Cat Tien National Park, southern Vietnam) / A.V. Tiunov (Editor). – M.: KMK Scientific Press. 2011. 277 р.101-124.
- Zryanin V. A., and M. V. Mokrousov. 2015. Contribution to the ant fauna of Lombok Island. Proceedings of the 10th ANeT International Conference, 23-26 October 2015, University of Kelaniya, Sri Lanka. 34



