Odontomachus haematodus
| Odontomachus haematodus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Odontomachus |
| Species: | O. haematodus |
| Binomial name | |
| Odontomachus haematodus (Linnaeus, 1758) | |
| Synonyms | |
| |
DaRocha et al. (2015) studied the diversity of ants found in bromeliads of a single large tree of Erythrina, a common cocoa shade tree, at an agricultural research center in Ilhéus, Brazil. Forty-seven species of ants were found in 36 of 52 the bromeliads examined. Bromeliads with suspended soil and those that were larger had higher ant diversity. Odontomachus haematodus was found in 15 different bromeliads and was associated with the suspended soil and litter of the plants.
| At a Glance | • Tandem running • Invasive |
Identification
A member of the Odontomachus haematodus group.
Identification Keys including this Taxon
Distribution
MacGown et al. (2014) - Due to misidentifications, the worldwide distribution of this species is not clearly understood at this time. However, based on recent examination of numerous specimens from the US, the distributional records for this species from this country can be considered reliable. Additionally, specimens from Brazil, British Guiana, and Peru were identified as O. haematodus by MacGown [LSAM, UGCA, and UMMZ]. Brown (1976) reported this species' distribution as continental South America from Orinoco Delta to Tucuma, Argentina. McGlynn (1999) reported O. haematodus as being introduced to Hawaii; however, these records have not been verified, nor is this species thought to have been established in that state. Krushelnycky et al. (2005) did not include O. haematodus in their recent list of ant species from Hawaii. In the US we have verified records of established populations of this species only from the Gulf Coast region, specifically from: Alabama: Baldwin, Escambia, and Mobile Counties. Florida: Escambia County. Louisiana: Orleans Parish. Mississippi: Greene and Jackson Counties. Distributional information for US specimens from AntWeb (2013) (Florida record), and examination of specimens in AUEM, MEM and USNM.
Odontomachus haematodus is native to South America. The earliest record in the US we found was of three specimens collected on 1 June 1956 from Mobile, Alabama. These specimens were borrowed from the USNM and examined by MacGown. Earliest MEM records are from 2000 from Baldwin County, Alabama, by which time this species had become locally abundant. Until recently, specimens of this species from the Gulf Coast were identified as Odontomachus insularis, Odontomachus brunneus, and/or Odontomachus ruginodis. However, after examination of workers and males of all three species, it became clear that this now-common Gulf Coast species is O. haematodus.
Latitudinal Distribution Pattern
Latitudinal Range: 21.678819° to -64.36°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States.
Neotropical Region: Argentina, Bahamas, Bolivia, Brazil, Colombia, Costa Rica, Ecuador, French Guiana, Greater Antilles, Grenada, Guadeloupe, Guatemala, Guyana, Honduras, Lesser Antilles, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Trinidad and Tobago, Venezuela.
Palaearctic Region: China.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
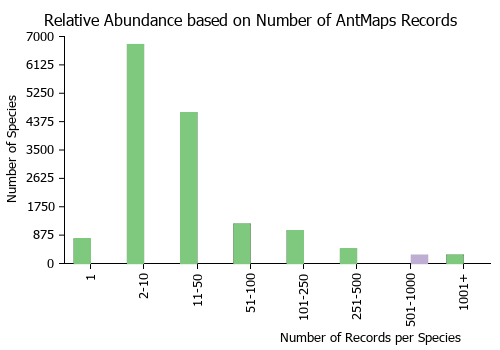
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |
|
Biology
While this species has been reported to nest in ant gardens (Campbell et al., 2022; Davidson, 1988)) it does not seem to be a true ant-garden taxon (i.e., one that is able to initiate ant gardens or is restricted to ant gardens) and is more likely to be a secondary resident or opportunistic ant-garden nester.
Regional Notes
French Guiana
A study by Talaga et al. (2015) investigated the species of ants found in the bromeliad Aechmea aquilega. Odontomachus haematodus was "by far the most frequent species in the rural area where it occupied 60% of all of the sampled plants sheltering ants. We noted that elementary nests (the colonies are composed of multiple nests) of this species were installed both between the leaves of several adjacent A. aquilega individuals and between their shoots and the bark of the host trees." They also note this species can nest in decaying logs and branches on the ground.
Puerto Rico
Wheeler (1908): Common, nesting under stones or logs or in untidy mound nests about the roots of trees, but only in shady places and rather rich soil.
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a host for the eucharitid wasp Chalcura deprivata (a parasite) (Universal Chalcidoidea Database) (primary host).
- This species is a host for the eucharitid wasp Kapala sp. (a parasitoid) (Quevillon, 2018) (multiple encounter modes; direct transmission; transmission outside nest).
- This species is a host for the eucharitid wasp Kapala terminalis (a parasite) (Universal Chalcidoidea Database) (primary host).
- This species is a host for the eucharitid wasp Schizaspidia convergens (a parasite) (Universal Chalcidoidea Database) (primary host).
- This species is a host for the phorid fly Apocephalus lopesi (a parasite) (Brown et al., 2015) (injured).
- This species is a host for the phorid fly Apocephalus lopesi (a parasite) (phorid.net) (attacked).
- This species is a prey for the phorid fly Dohrniphora longirostrata (a predator) (Quevillon, 2018).
- This species is a prey for the phorid fly Dohrniphora sp. (a predator) (Quevillon, 2018).
- This species is a host for the phorid fly Dohrniphora sp. (longi-gp_females (a parasite) (Brown et al., 2015) (injured).
- This species is a host for the nematode Mermithidae (unspecified "Mermix") (a parasite) in Neotropics (Wheeler, 1928; Laciny, 2021).
Life History Traits
- Mean colony size: 500 (Holldobler & Engel, 1978; Beckers et al., 1989)
- Foraging behaviour: tandem recruitment (Holldobler & Engel, 1978; Beckers et al., 1989)
Castes
Worker
 
| |
| . | |
Images from AntWeb
   
| |
| Worker. Specimen code casent0173537. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ALWC, Alex L. Wild Collection. |
Male
Images from AntWeb
    
| |
| Worker. Specimen code casent0103105. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- haematodus. Formica haematoda Linnaeus, 1758: 582 (q.) (no state data).
- Type-material: holotype queen.
- Type-locality: none stated, "Habitat in America meridionali" (Rolander); probably Suriname: Paramaribo.
- Type-depository: ZMLS.
- [Misspelled as haematodes by Lepeletier de Saint-Fargeau, 1835: 187, Mayr, 1862: 711, André, 1887: 290, Emery, 1887b: 428, and many others.]
- André, 1887: 290 (m.); Emery, 1899c: 5 (l.); Wheeler, W.M. 1900b: 16 (l.); Eidmann, 1944: 437 (l.); Wheeler, G.C. & Wheeler, J. 1952c: 646 (l.); MacGown, et al. 2014: 539 (m.).
- Combination in Myrmecia: Fabricius, 1804: 425; Gravenhorst, 1807: 287;
- combination in Odontomachus: Latreille, 1804: 179; Lepeletier de Saint-Fargeau, 1835: 187.
- Status as species: Linnaeus, 1767: 965; De Geer, 1773: 601; Fabricius, 1775: 395; Fabricius, 1782: 494; Retzius, 1783: 75; Fabricius, 1787: 311; Gmelin, 1790: 2803; Christ, 1791: 516; Olivier, 1792: 502; Fabricius, 1793: 364; Latreille, 1802c: 192; Fabricius, 1804: 425; Latreille, 1804: 179; Gravenhorst, 1807: 287; Latreille, 1818c: 570; Lepeletier de Saint-Fargeau, 1835: 187; Smith, F. 1858b: 76; Gerstäcker, 1859: 262; Roger, 1861a: 24; Smith, F. 1862b: 31; Mayr, 1862: 711; Gerstäcker, 1862: 503; Roger, 1863b: 22; Mayr, 1863: 436; Mayr, 1865: 63; Mayr, 1867a: 79 (redescription); Mayr, 1872: 148; Mayr, 1876: 85; Emery, 1878a: ix (in list); André, 1884b: 538; Mayr, 1884: 33; Mayr, 1886d: 437; Cresson, 1887: 258; André, 1887: 290; Emery, 1887b: 428; Emery, 1890a: 65; Emery, 1890b: 44; Forel, 1891b: 104 (redescription); Cameron, 1891: 93; André, 1892b: 52; Emery, 1892d: 557, 561 (in key); Forel, 1893g: 353; André, 1893b: 152; Dalla Torre, 1893: 50; Emery, 1893e: 190; Emery, 1893f: 243; Emery, 1893g: 262; Forel, 1894b: 74; Emery, 1894k: 50; Emery, 1895h: 22; Mayr, 1895: 125; André, 1895a: 3; Forel, 1895b: 118; Mayr, 1896: 238; Pergande, 1896: 873; Mayr, 1897: 424; Emery, 1897d: 557; Wasmann, 1897: 250; Forel, 1899c: 20; Emery, 1899a: 500; Emery, 1899c: 5; Forel, 1900c: 58; Emery, 1900d: 672; Wheeler, W.M. 1900b: 2; Forel, 1901b: 5; Forel, 1901h: 81; Dahl, 1901: 12; Emery, 1901f: 113; Emery, 1901g: 566; Bingham, 1903: 47; Forel, 1903d: 399; Emery, 1904b: 591; Ruzsky, 1905b: 759; Forel, 1905c: 8; Wheeler, W.M. 1905b: 122; Emery, 1906c: 118; Forel, 1907e: 1; Forel, 1908a: 2; Forel, 1908b: 35; Wheeler, W.M. 1908a: 126; Wheeler, W.M. 1908b: 159; Wheeler, W.M. 1909b: 231; Wheeler, W.M. 1909d: 339; Forel, 1909b: 51; Forel, 1909d: 221; Santschi, 1910c: 350; Stitz, 1910: 130; Forel, 1910f: 4; Emery, 1911b: 531; Emery, 1911d: 114; Wheeler, W.M. 1911a: 22; Wheeler, W.M. 1911b: 168; Forel, 1911b: 193; Forel, 1911i: 215; Stitz, 1911a: 357; Wheeler, W.M. 1912a: 45; Forel, 1912d: 97; Forel, 1912j: 179; Forel, 1912k: 159; Forel, 1913a: 108; Forel, 1913b: 309; Forel, 1913h: 347; Forel, 1913k: 159; Wheeler, W.M. 1913d: 239; Santschi, 1914b: 58; Santschi, 1914d: 331; Emery, 1914b: 180; Emery, 1914f: 400; Bruch, 1914: 213; Viehmeyer, 1914c: 516; Wheeler, W.M. & Mann, 1914: 16; Arnold, 1915: 108; Stitz, 1916: 373; Mann, 1916: 418; Wheeler, W.M. 1916c: 3; Wheeler, W.M. 1916d: 323; Viehmeyer, 1916a: 116; Crawley, 1916b: 367; Gallardo, 1918b: 99; Wheeler, W.M. 1919e: 60; Mann, 1919: 303; Mann, 1920: 404; Santschi, 1920i: 2; Mann, 1921: 427; Mann, 1922: 18; Wheeler, W.M. 1922a: 102, 793, 1013; Wheeler, W.M. 1922c: 4; Borgmeier, 1923: 78; Viehmeyer, 1923: 87; Wheeler, W.M. 1923a: 3; Wheeler, W.M. 1924b: 243; Crawley, 1924: 388; Stitz, 1925: 115; Mann, 1925b: 5; Karavaiev, 1925c: 293; Wheeler, W.M. & Chapman, 1925: 71; Wheeler, W.M. 1925a: 10; Karavaiev, 1926d: 422; Menozzi, 1927c: 267; Wheeler, W.M. 1927d: 2; Wheeler, W.M. 1927h: 86; Santschi, 1928a: 44; Santschi, 1928c: 68; Santschi, 1928h: 124; Wheeler, W.M. 1929f: 2; Wheeler, W.M. 1929g: 37; Karavaiev, 1930a: 212; Santschi, 1930b: 60; Menozzi, 1930b: 80; Menozzi, 1930d: 327; Stärcke, 1930: 373; Wheeler, W.M. 1930h: 61; Aguayo, 1932: 216; Kutter, 1932: 207; Santschi, 1932b: 13; Stitz, 1932a: 367; Wheeler, W.M. 1932d: 14; Menozzi, 1933a: 101; Wheeler, W.M. 1934a: 173; Karavaiev, 1935a: 75; Santschi, 1935b: 263; Wheeler, W.M. 1935g: 15; Wheeler, W.M. 1936f: 5; Smith, M.R. 1937: 827; Wheeler, W.M. 1937a: 22; Wheeler, W.M. 1938: 251; Finzi, 1939a: 154; Santschi, 1939f: 160; Teranishi, 1940: 64; Menozzi, 1942: 166; Weber, 1943c: 303; Santschi, 1937b: 95; Donisthorpe, 1943d: 445; Eidmann, 1944: 437, 469; Stärcke, 1944b: xviii; Donisthorpe, 1947c: 579; Donisthorpe, 1948b: 303; Donisthorpe, 1949g: 408; Creighton, 1950a: 55; Chapman & Capco, 1951: 43; Menozzi & Consani, 1952: 61; Bernard, 1953b: 214; Kusnezov, 1953b: 336; Kempf, 1961b: 497; Kempf, 1962b: 17 (in key); Prins, 1963: 100; Baltazar, 1966: 239; Kempf, 1970b: 327; Kempf, 1972a: 170; Kempf & Lenko, 1976: 60; Brown, 1976a: 104, 148; Brandão, 1991: 363; Bolton, 1995b: 295; Tiwari, 1999: 21; Mathew & Tiwari, 2000: 289; Zhou, 2001b: 27; Zhou, 2006: 579; Wild, 2007b: 39; Rodriguez, J. 2008: 163; Zhou & Ran, 2010: 106; Guénard & Dunn, 2012: 60; MacGown, et al. 2014: 536 (redescription); Bezděčková, et al. 2015: 124; Feitosa, 2015c: 99; Deyrup, 2017: 29; Fernández & Guerrero, 2019: 539.
- Senior synonym of brunneipes: Brown, 1976a: 104; Brandão, 1991: 364; Zhou, 2001b: 27; MacGown, et al. 2014: 537.
- Senior synonym of hirsutiusculus: Roger, 1861a: 24; Roger, 1863b: 22; Mayr, 1863: 437; Mayr, 1865: 64; Mayr, 1872: 148; Forel, 1891b: 104; Brown, 1976a: 104; Brandão, 1991: 364; Bolton, 1995b: 295; Zhou, 2001b: 27; MacGown, et al. 2014: 537.
- Senior synonym of maxillosa: Retzius, 1783: 75; Gmelin, 1790: 2803; Olivier, 1792: 502; Latreille, 1802c: 192; Lepeletier de Saint-Fargeau, 1835: 187; Smith, F. 1858b: 76; Gerstäcker, 1862: 503; Mayr, 1863: 437; Roger, 1863b: 22; Mayr, 1865: 63; Mayr, 1872: 148; Forel, 1891b: 104; Dalla Torre, 1893: 50; Wheeler, W.M. 1908a: 125; Emery, 1911d: 114; Arnold, 1915: 108; Gallardo, 1918b: 99; Wheeler, W.M. 1919e: 60; Wheeler, W.M. 1922a: 793; Borgmeier, 1923: 78; Kempf, 1972a: 170; Brown, 1976a: 104; Bolton, 1995b: 295; Zhou, 2001b: 27; MacGown, et al. 2014: 536.
- Senior synonym of pallipes: Brown, 1976a: 104; Brandão, 1991: 364; Bolton, 1995b: 295; Zhou, 2001b: 27; MacGown, et al. 2014: 537.
- Distribution: Argentina, Bolivia, Brazil, Colombia, Ecuador, Guyana, Paraguay, Peru, Suriname, U.S.A., Venezuela.
- [Note: Afrotropical records of haematodus prior to Brown, 1976a: 167, are correctly referred to troglodytes. Oriental, Malesian, and Austral records of haematodus prior to Brown, 1976a: 167, are almost entirely misidentifications of simillimus.]
- brunneipes. Odontomachus haematoda var. brunneipes Menozzi, 1935b: 191.
- Type-material: holotype worker.
- Type-locality: Brazil: Pará, Chiriqui.
- Type-depository: MSNG.
- [First available use of Odontomachus haematodus r. pubescens var. bruneipes Emery, 1893d: 91 (footnote) (w.) BRAZIL (Pará); unavailable (infrasubspecific) name (Bolton, 1995b: 295).]
- [Misspelled as beuneipes by MacGown, et al. 2014: 537.]
- As unavailable (infrasubspecific) name: Emery, 1911d: 115; Borgmeier, 1923: 79; Eidmann, 1936a: 37; Kempf, 1972a: 171.
- Junior synonym of haematodus: Brown, 1976a: 104; Brandão, 1991: 364; Zhou, 2001b: 27; MacGown, et al. 2014: 537.
- hirsutiusculus. Odontomachus hirsutiusculus Smith, F. 1858b: 78 (w.) BRAZIL (Pará).
- Type-material: 2 syntype workers.
- Type-locality: Brazil: Santarem, 54/63 (H.W. Bates).
- [Note BMNH Accessions Register adds, “1854 no. 63 (Oct. 11) Brazil (Santarem on the Amazon). Purchased from Stevens. Collected by Mr Bates, Alta de Chai near Santarem.”]
- Type-depository: BMNH.
- [Misspelled as hushirsutiusculus by Zhou, 2001b: 27.]
- Forel, 1909a: 252 (q.).
- As unavailable (infrasubspecific) name: Forel, 1899c: 20; Forel, 1909a: 252; Wheeler, W.M. 1905b: 122; Emery, 1911d: 115; Forel, 1912c: 28 (in text); Forel, 1913l: 205; Wheeler, W.M. 1916d: 323; Wheeler, W.M. 1922c: 4; Borgmeier, 1923: 79; Santschi, 1939f: 160; Kempf, 1972a: 171.
- Subspecies of insularis: Emery, in Dalla Torre, 1893: 51.
- Subspecies of haematodus: Roger, 1861a: 24; Emery, 1890b: 44 (footnote); Forel, 1893g: 354; Emery, 1896h: 625; Forel, 1907e: 1; Forel, 1908b: 35; Wheeler, W.M. 1911a: 22; Emery, 1911d: 115; Luederwaldt, 1918: 36.
- Junior synonym of haematodus: Roger, 1861a: 24; Roger, 1863b: 22; Mayr, 1863: 437; Mayr, 1865: 64; Mayr, 1872: 148; Forel, 1891b: 104; Brown, 1976a: 104; Brandão, 1991: 364; Bolton, 1995b: 296; Zhou, 2001b: 27; MacGown, et al. 2014: 537.
- maxillosa. Formica maxillosa De Geer, 1773: 601, pl. 31, figs. 3-5 (q.) SURINAME.
- Type-material: holotype queen.
- Type-locality: Suriname: (no further data) (Rolander).
- Type-depository: MNHN.
- Junior synonym of haematodus: Retzius, 1783: 75; Gmelin, 1790: 2803; Olivier, 1792: 502; Latreille, 1802c: 192; Lepeletier de Saint-Fargeau, 1835: 187; Smith, F. 1858b: 76; Gerstäcker, 1862: 503; Mayr, 1863: 437; Roger, 1863b: 22; Mayr, 1865: 63; Mayr, 1872: 148; Forel, 1891b: 104; Dalla Torre, 1893: 50; Wheeler, W.M. 1908a: 125; Emery, 1911d: 114; Arnold, 1915: 108; Gallardo, 1918b: 99; Wheeler, W.M. 1919e: 60; Wheeler, W.M. 1922a: 793; Borgmeier, 1923: 78; Kempf, 1972a: 170; Brown, 1976a: 104; Bolton, 1995b: 296; Zhou, 2001b: 27; MacGown, et al. 2014: 536.
- pallipes. Odontomachus haematoda var. pallipes Crawley, 1916b: 368 (w.) GUYANA.
- Type-material: syntype workers (number not stated).
- Type-locality: Guyana (“British Guiana”): (no further data), 20.iv.1915 (G.E. Bodkin).
- Type-depository: OXUM (perhaps also in BMNH).
- Subspecies of haematodus: Kempf, 1972a: 170.
- Junior synonym of haematodus: Brown, 1976a: 104; Brandão, 1991: 364; Bolton, 1995b: 296; Zhou, 2001b: 27; MacGown, et al. 2014: 537.
The following notes on F. Smith type specimens have been provided by Barry Bolton (details):
Odontomachus hirsutiusculus
Two syntype workers in The Natural History Museum. Both labelled, “Santarem. 54/63.” Acc. Reg.: “1854 no. 63 (Oct. 11) Brazil (Santarem on the Amazon). Purchased from Stevens. Collected by Mr Bates, Alta de Chai near Santarem.”
Description
Karyotype
- n = 22, 2n = 44, karyotype = 8SM+18ST+18A (44A) (French Guiana) (Santos et al., 2007; Mariano et al., 2015; Aguiar et al., 2020; Teixeira et al., 2020).
References
- Aguiar, H.J.A.C., Barros, L.A.C., Silveira, L.I., Petitclerc, F., Etienne, S., Orivel, J. 2020. Cytogenetic data for sixteen ant species from North-eastern Amazonia with phylogenetic insights into three subfamilies. Comparative Cytogenetics 14(1): 43–60 (doi:10.3897/CompCytogen.v14i1.46692).
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Azevedo Filho, P.A.de, Vasconcelos, F.R., Santos, R.C.G.dos, Morais, S.M.de. 2021. Cuticular hydrocarbons from ants (Hymenoptera: Formicidae) Odontomachus bauri (Emery) from the tropical forest of Maranguape, Ceará, Brazil. Research, Society and Development 10, e13010817119 (doi:10.33448/rsd-v10i8.17119).
- Beckers R., Goss, S., Deneubourg, J.L., Pasteels, J.M. 1989. Colony size, communication and ant foraging Strategy. Psyche 96: 239-256 (doi:10.1155/1989/94279).
- Borowiec, L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Brown, W. L., Jr. 1976c. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section A. Introduction, subtribal characters. Genus Odontomachus. Stud. Entomol. 19: 67-171 (page 104, Senior synonym of hirsutiusculus, Senior synonym of pallipes, and material of the unavailable name bruneipes referred here)
- Camargo, K.S. de. 2011. Composicao e diversidade de "Poneromorfas" (Hymenoptera, Formicidae) em duas fitofisionomias de cerrado e padroes de distribuicao de "Poneromorfas", Pseudomyrmecinae e Cephalotini (Myrmicinae) para o Brasil. Thesis, Universidade de Brasilia.
- Campbell, L.C.E., Kiers, E.T., Chomicki, G. 2022. The evolution of plant cultivation by ants. Trends in Plant Science (doi:10.1016/j.tplants.2022.09.005).
- DaRocha, W. D., S. P. Ribeiro, F. S. Neves, G. W. Fernandes, M. Leponce, and J. H. C. Delabie. 2015. How does bromeliad distribution structure the arboreal ant assemblage (Hymenoptera: Formicidae) on a single tree in a Brazilian Atlantic forest agroecosystem? Myrmecological News. 21:83-92.
- Davidson, D.W. 1988. Ecological studies of neotropical ant gardens. Ecology 69: 1138-1152.
- Emery, C. 1899g. Intorno alle larve di alcune formiche. Mem. R. Accad. Sci. Ist. Bologna (5) 8: 3-10 (page 5, larva described)
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Houadria, M., Menzel, F. 2021. Digging Deeper into the Ecology of Subterranean Ants: Diversity and Niche Partitioning across Two Continents. Diversity 13, 53 (doi:10.3390/d13020053).
- Ladino, N., Feitosa, R.M. 2022. Ants (Hymenoptera: Formicidae) of the Parque Estadual São Camilo, an isolated Atlantic Forest remnant in western Paraná, Brazil. ZOOLOGIA 39: e22001 (doi:10.1590/S1984-4689.v39.e22001).
- Latreille, P. A. 1802b. Histoire naturelle générale et particulière des Crustacés et des insectes. Tome 3. Familles naturelles des genres. Paris: F. Dufart, xii + 467 pp. (page 192, Senior synonym of maxillosa)
- Latreille, P. A. 1804. Tableau méthodique des insectes. Pp. 129-200 in: Société de Naturalistes et d'Agriculteurs. Nouveau dictionnaire d'histoire naturelle. Tome 24. Paris: Déterville, 84 + 85 + 238 + 18 + 34 pp. (page 179, Combination in Odontomachus)
- Lin, T.-H., Chan, K.-W., Hsu, F.-C., Lin, C.-C., Tseng, H.-Y. 2023. Putative source and niche shift pattern of a new alien ant species (Odontomachus troglodytes) in Taiwan. PeerJ 11, e14718 (doi:10.7717/peerj.14718).
- Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Holmiae [= Stockholm]: L. Salvii, 824 pp. (page 582, queen described)
- Lubertazzi, D. 2019. The ants of Hispaniola. Bulletin of the Museum of Comparative Zoology, 162(2), 59-210 (doi:10.3099/mcz-43.1).
- MacGown, J.A., Boudinot, B., Deyrup, M. & Sorger, D.M. 2014. A review of the Nearctic Odontomachus (Hymenoptera: Formicidae: Ponerinae) with a treatment of the males. Zootaxa 3802(4): 515-552.
- Mariano, C.S.F., Santos, I.S., Silva, J.G., Costa, M.A., Pompolo, S.G. 2015. Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie, J.H.C., Feitosa, R.M., Serrao, J.E., Mariano, C.S.F., Majer, J.D. (eds) As formigas poneromorfas do Brasil, 1st edn. Ilhéus, Brasil, pp 102–125 (doi:10.7476/9788574554419.0010).
- Melo, T.S., Koch, E.B.A., Andrade, A.R.S., Travassos, M.L.O., Peres, M.C.L., Delabie, J.H.C. 2021. Ants (Hymenoptera: Formicidae) in different green areas in the metropolitan region of Salvador, Bahia state, Brazil. Brazilian Journal of Biology 82, e236269 (doi:10.1590/1519-6984.236269).
- Meurgey, F. 2020. Challenging the Wallacean shortfall: A total assessment of insect diversity on Guadeloupe (French West Indies), a checklist and bibliography. Insecta Mundi 786: 1–183.
- Moura, M.N., Cardoso, D.C., Cristiano, M.P. 2020. The tight genome size of ants: diversity and evolution under ancestral state reconstruction and base composition. Zoological Journal of the Linnean Society, zlaa135 (doi:10.1093/zoolinnean/zlaa135).
- Olivier, A. G. 1792. Encyclopédie méthodique. Histoire naturelle. Insectes. Tome 6. (pt. 2). Paris: Panckoucke, pp. 369-704. (page 502, Senior synonym of maxillosa)
- Retzius, A. J. 1783. Caroli de Geer. Genera et species insectorum e generosissimi auctoris scriptis extraxit, digessit, Latine quoad partem reddidit, et terminologiam insectorum Linneanam addidit. Lipsiae [= Leipzig]: Cruse, 220 pp. (page 75, Senior synonym of maxillosa)
- Roger, J. 1863b. Verzeichniss der Formiciden-Gattungen und Arten. Berl. Entomol. Z. 7(B Beilage: 1-65 (page 22, Senior synonym of hirsutiusculus)
- Rosas-Mejía, M., Guénard, B., Aguilar-Méndez, M. J., Ghilardi, A., Vásquez-Bolaños, M., Economo, E. P., Janda, M. 2021. Alien ants (Hymenoptera: Formicidae) in Mexico: the first database of records. Biological Invasions 23(6), 1669–1680 (doi:10.1007/s10530-020-02423-1).
- SANTOS, I.S.; COSTA, M.A., MARIANO, C.F.S.; DELABIE, J.H.C.; SILVA, J.G. Análise citogenética em Odontomachus haematodus (Hymenoptera: Formicidae) de ocorrência no corredor central da Mata Atlântica. In: CONGRESSO BRASILEIRO DE GENÉTICA, 53, 2007, Águas de Lindoia. Resumos do 53º Congresso Brasileiro de Genética, Águas de Lindoia -SP, 2007, 53.
- Satria, R. 2017. Taxonomy of the ant genus Odontomachus (Hymenoptera: Formicidae: Ponerinae) in the Indo-Chinese and Indo-Malayan subregions. Ph.D. thesis, Tokyo Metropolitan University.
- Subedi, I.P., Budha, P.B., Bharti, H., Alonso, L., Yamane, S. 2023. Ponerine ants of Nepal (Hymenoptera: Formicidae, Ponerinae): a generic synopsis, new faunal records, and rediscovery of a rare ant, Emeryopone franzi (Baroni Urbani 1975). (doi:10.20362/am.016003).
- Talaga, S., J. H. C. Delabie, O. Dezerald, A. Salas-Lopez, F. Petitclerca, C. Leroy, B. Heraultd, R. Cereghino, and A. Dejean. 2015. A bromeliad species reveals invasive ant presence in urban areas of French Guiana. Ecological Indicators. 58:1-7. doi:10.1016/j.ecolind.2015.05.027
- Teixeira, G.A., Barros, L.A.C., Lopes, D.M., Aguiar, H.J.A.C.de 2020. Karyotypic data of five ant taxa from the Brazilian Atlantic rainforest. Sociobiology 67, 604. (doi:10.13102/sociobiology.v67i4.5833).
- Touchard, A., Dejean, A., Escoubas, P., Orivel, J. 2015. Intraspecific variations in the venom peptidome of the ant Odontomachus haematodus (Formicidae: Ponerinae) from French Guiana. Journal of Hymenoptera Research 47, 87–101 (doi:10.3897/jhr.47.6804).
- Ulysséa, M.A., Brandão, C.R.F. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia 57, 217–224 (doi:10.1590/s0085-56262013005000002).
- Varela-Hernández, F., Medel-Zosayas, B., Martínez-Luque, E.O., Jones, R.W., De la Mora, A. 2020. Biodiversity in central Mexico: Assessment of ants in a convergent region. Southwestern Entomologist 454: 673-686.
- Wetterer, J.K. 2021. Ants (Hymenoptera, Formicidae) of St. Vincent, West Indies. Sociobiology 68, e6725 (doi:10.13102/sociobiology.v68i2.6725).
- Wheeler, G. C.; Wheeler, J. 1952c. The ant larvae of the subfamily Ponerinae - Part II. Am. Midl. Nat. 48: 604-672 (page 646, larva described)
- Wheeler, W. M. 1908a. The ants of Porto Rico and the Virgin Islands. Bull. Am. Mus. Nat. Hist. 24: 117-158.
- Wheeler, W.M. 1928. Mermis parasitism and intercastes among ants. Journal of Experimental Zoology 50: 165-237 (doi:10.1002/jez.1400500202).
References based on Global Ant Biodiversity Informatics
- Aguayo C. G. 1932. Notes on West Indian ants. Bulletin of the Brooklyn Entomological Society 27: 215-227.
- Alonso L. E., J. Persaud, and A. Williams. 2016. Biodiversity assessment survey of the south Rupununi Savannah, Guyana. BAT Survey Report No.1, 306 pages.
- André E. 1893. Description de quatre espèces nouvelles de fourmis d'Amérique. Rev. Entomol. (Caen) 12: 148-152.
- Bezdeckova K., P. Bedecka, and I. Machar. 2015. A checklist of the ants (Hymenoptera: Formicidae) of Peru. Zootaxa 4020 (1): 101–133.
- Bezerra de Carvalho M., and A. de Oliveira Freitas. 1960. Terceira contribuicao para o catalogo dos insetos de Pernambuco. Arq. Inst. Pesq. Agron. (Recife) 2: 27-60.
- Bieber A. G. D., O. P. G. Darrault, C. da Costa Ramos, K. K. Melo, and I. R. Leal. 2006. Formigas. p.244-262. In Porto K L, Tabarelli M, Almeida-Cortez J (eds) Diversidade biológica e conservação da Floresta Atlântica ao norte do rio São Francisco. Recife, Editora Universitária da UFPE, 363p
- Blüthgen, N., M. Verhaagh, W. Goitia and N. Bluthgen. 2000. Ant nests in tank bromeliads an example of non-specific interaction. Insectes Sociaux 47:313-316
- Borgmeier T. 1923. Catalogo systematico e synonymico das formigas do Brasil. 1 parte. Subfam. Dorylinae, Cerapachyinae, Ponerinae, Dolichoderinae. Archivos do Museu Nacional (Rio de Janeiro) 24: 33-103.
- Brandao, C.R.F. 1991. Adendos ao catalogo abreviado das formigas da regiao neotropical (Hymenoptera: Formicidae). Rev. Bras. Entomol. 35: 319-412.
- Brown W. L., Jr. 1976. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section A. Introduction, subtribal characters. Genus Odontomachus. Stud. Entomol. 19: 67-171.
- Bruch C. 1914. Catálogo sistemático de los formícidos argentinos. Revista del Museo de La Plata 19: 211-234.
- Cheng D., Z. Chen, and S. Zhou. 2015. An analysis on the ant fauna of Jinzhongshan Nature Reserve in Gunagxi, China. Journal of Guangxi Normal University: Natural Science Edition 33(3): 129.137.
- Cividanes F. J., J. C. Barbosa, I. C. F. Martins, F. Pattaro, M. A. Nunes, R. Souza Santos. 2009. Diversity and spatial distribution of ground arthropods in agroecosystems. Bragantia, Campinas, 68(4): 991-1002.
- Corassa J. N., I. C. Magistrali, J. C. Moreno, E. B. Cantarelli, and A. Corassa. Effect of formicid granulated baits on non-target ants biodiversity in eucalyptus plantations litter. Comunicata Scientiae 4(1): 35-42.
- Costa-Milanez C. B., G. Lourenco-Silva, P. T. A. Castro, J. D. Majer, and S. P. Ribeiro. 2014. Are ant assemblages of Brazilian veredas characterised by location or habitat type? Braz. J. Biol. 74(1): 89-99.
- Crawley W. C. 1916. Ants from British Guiana. Ann. Mag. Nat. Hist. 8(17): 366-378.
- Cuezzo, F. 1998. Formicidae. Chapter 42 in Morrone J.J., and S. Coscaron (dirs) Biodiversidad de artropodos argentinos: una perspectiva biotaxonomica Ediciones Sur, La Plata. Pages 452-462.
- Dad J. M., S. A. Akbar, H. Bharti, and A. A. Wachkoo. 2019. Community structure and ant species diversity across select sites ofWestern Ghats, India. Acta Ecologica Sinica 39: 219–228.
- Emery C. 1890. Voyage de M. E. Simon au Venezuela (Décembre 1887 - Avril 1888). Formicides. Annales de la Société Entomologique de France (6)10: 55-76.
- Emery C. 1896. Formiciden, gesammelt in Paraguay von Dr. J. Bohls. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 9: 625-638.
- Emery C. 1899. Intorno alle larve di alcune formiche. Mem. R. Accad. Sci. Ist. Bologna (5) 8: 3-10.
- Emery C. 1906. Studi sulle formiche della fauna neotropica. XXVI. Bullettino della Società Entomologica Italiana 37: 107-194.
- Escalante Gutiérrez J. A. 1993. Especies de hormigas conocidas del Perú (Hymenoptera: Formicidae). Revista Peruana de Entomología 34:1-13.
- Escalante J. A. 1975. Hormigas de la Provincia de la Convencionm Cusco. Revista Peruana de Entomologia 18:125-126.
- Escalante J. A. 1976. Hormigas del valle de K'Osnipata (Paucartambo, Cusco). Revista Peruana de Entomologia 107-108.
- Fernandes I., and J. de Souza. 2018. Dataset of long-term monitoring of ground-dwelling ants (Hymenoptera: Formicidae) in the influence areas of a hydroelectric power plant on the Madeira River in the Amazon Basin. Biodiversity Data Journal 6: e24375.
- Fernández F. 2008. Subfamilia Ponerinae s.str. Pp. 123-218 in: Jiménez, E.; Fernández, F.; Arias, T.M.; Lozano-Zambrano F. H. (eds.) 2008. Sistemática, biogeografía y conservación de las hormigas cazadoras de Colombia. Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, xiv + 609 pp.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Fichaux M., B. Bechade, J. Donald, A. Weyna, J. H. C. Delabie, J. Murienne, C. Baraloto, and J. Orivel. 2019. Habitats shape taxonomic and functional composition of Neotropical ant assemblages. Oecologia 189(2): 501-513.
- Forel A. 1901. Variétés myrmécologiques. Annales de la Société Entomologique de Belgique 45: 334-382.
- Forel A. 1907. Formiciden aus dem Naturhistorischen Museum in Hamburg. II. Teil. Neueingänge seit 1900. Mitt. Naturhist. Mus. Hambg. 24: 1-20.
- Forel A. 1908. Ameisen aus Sao Paulo (Brasilien), Paraguay etc. gesammelt von Prof. Herm. v. Ihering, Dr. Lutz, Dr. Fiebrig, etc. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 58: 340-418.
- Forel A. 1908. Fourmis de Costa-Rica récoltées par M. Paul Biolley. Bulletin de la Société Vaudoise des Sciences Naturelles 44: 35-72.
- Forel A. 1913. Fourmis d'Argentine, du Brésil, du Guatémala & de Cuba reçues de M. M. Bruch, Prof. v. Ihering, Mlle Baez, M. Peper et M. Rovereto. Bulletin de la Société Vaudoise des Sciences Naturelles. 49: 203-250.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Galkowski C. 2016. New data on the ants from the Guadeloupe (Hymenoptera, Formicidae). Bull. Soc. Linn. Bordeaux 151, 44(1): 25-36.
- Gibernau M., J. Orivel, J. H. C. Delabie, D. Barabe, and A. Dejean. 2007. An asymmetrical relationship between an arboreal ponerine ant and a trash-basket epiphyte (Araceae). Biological Journal of the Linnean Society 91: 341-346.
- Gomes E. C. F., G. T. Ribeiro, T. M. S. Souza, and L. Sousa-Souto. 2014. Ant assemblages (Hymenoptera: Formicidae) in three different stages of forest regeneration in a fragment of Atlantic Forest in Sergipe, Brazil. Sociobiology 61(3): 250-257.
- Groc S., J. H. C. Delabie, F. Fernandez, F. Petitclerc, B. Corbara, M. Leponce, R. Cereghino, and A. Dejean. 2017. Litter-dwelling ants as bioindicators to gauge the sustainability of small arboreal monocultures embedded in the Amazonian rainforest. Ecological Indicators 82: 43-49.
- Groc S., J. H. C. Delabie, F. Fernandez, M. Leponce, J. Orivel, R. Silvestre, Heraldo L. Vasconcelos, and A. Dejean. 2013. Leaf-litter ant communities (Hymenoptera: Formicidae) in a pristine Guianese rainforest: stable functional structure versus high species turnover. Myrmecological News 19: 43-51.
- Guénard B., and R. R. Dunn. 2012. A checklist of the ants of China. Zootaxa 3558: 1-77.
- Houadria M., A. Salas-Lopez, J. Orivel, N. Bluthgen, and F. Menzel. 2015. Dietary and temporal niche differentiati on in tropical ants—can they explain local ant coexistence? Biotropica 47(2): 208-217.
- IZIKO South Africa Museum Collection
- Jaffe, K., Mauleon, H. and Kermarrec A. 1990. Predatory Ants of Diaprepes Abbreviatus (Coleoptera: Curculionidae) In Citrus Groves In Martinique and Guadeloupe, F.W.I. Florida Entomologist. 73(4):684-687.
- Kempf W. W. 1961. A survey of the ants of the soil fauna in Surinam (Hymenoptera: Formicidae). Studia Entomologica 4: 481-524.
- Kempf W. W., and K. Lenko. 1976. Levantamento da formicifauna no litoral norte e ilhas adjacentes do Estado de São Paulo, Brasil. I. Subfamilias Dorylinae, Ponerinae e Pseudomyrmecinae (Hym., Formicidae). Studia Entomologica 19: 45-66.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Kusnezov N. 1963. Zoogeografia de las hormigas en sudamerica. Acta Zoologica Lilloana 19: 25-186
- Kusnezov N. 1978. Hormigas argentinas: clave para su identificación. Miscelánea. Instituto Miguel Lillo 61:1-147 + 28 pl.
- Leponce M., J. H. C. Delabie, J. Orivel, J. Jacquemin, M. Calvo Martin, and A. Dejean. 2019. Tree-dwelling ant survey (Hymenoptera, Formicidae) in Mitaraka, French Guiana, in Touroult J. (ed.), “Our Planet Reviewed” 2015 large-scale biotic survey in Mitaraka, French Guiana. Zoosystema 41 (10): 163-179.
- Luederwaldt H. 1918. Notas myrmecologicas. Rev. Mus. Paul. 10: 29-64.
- MacGown J. A., B. Boudinot, M. Deyrup, and D. M. Sorger. 2014. A review of the Nearctic Odontomachus (Hymenoptera: Formicidae: Ponerinae) with a treatment of the males. Zootaxa 3802(4): 515-552.
- Mann W. M. 1916. The Stanford Expedition to Brazil, 1911, John C. Branner, Director. The ants of Brazil. Bulletin of the Museum of Comparative Zoology 60: 399-490
- Medina U. C. A., F. Fernandez, and M. G. Andrade-C. 2010. Insectos: escarabajos coprofagos, hormigas y mariposas. Capitulo 6. Pp 197-215. En: Lasso, C. A., J. S. Usma, F. Trujillo y A. Rial (eds.). 2010. Biodiversidad de la cuenca del Orinoco: bases científicas para la identificación de áreas prioritarias para la conservación y uso sostenible de la biodiversidad. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, WWF Colombia, Fundación Omacha, Fundación La Salle e Instituto de Estudios de la Orinoquia (Universidad Nacional de Colombia). Bogotá, D. C., Colombia.
- Nascimento Santos M., J. H. C. Delabie, and J. M. Queiroz. 2019. Biodiversity conservation in urban parks: a study of ground-dwelling ants (Hymenoptera: Formicidae) in Rio de Janeiro City. Urban Ecosystems https://doi.org/10.1007/s11252-019-00872-8
- Pereira M. C., J. H. C. Delabie, Y. R. Suarez, and W. F. Antonialli Junior. 2013. Spatial connectivity of aquatic macrophytes and flood cycle influence species richness of an ant community of a Brazilian floodplain. Sociobiology 60(1): 41-49.
- Pignalberi C. T. 1961. Contribución al conocimiento de los formícidos de la provincia de Santa Fé. Pp. 165-173 in: Comisión Investigación Científica; Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina) 1961. Actas y trabajos del primer Congreso Sudamericano de Zoología (La Plata, 12-24 octubre 1959). Tomo III. Buenos Aires: Librart, 276 pp.
- Pires de Prado L., R. M. Feitosa, S. Pinzon Triana, J. A. Munoz Gutierrez, G. X. Rousseau, R. Alves Silva, G. M. Siqueira, C. L. Caldas dos Santos, F. Veras Silva, T. Sanches Ranzani da Silva, A. Casadei-Ferreira, R. Rosa da Silva, and J. Andrade-Silva. 2019. An overview of the ant fauna (Hymenoptera: Formicidae) of the state of Maranhao, Brazil. Pap. Avulsos Zool. 59: e20195938.
- Resende J. J., G. M. de M. Santos, I. C. do Nascimento, J. H. C. Delabie, and E. M. da Silva. 2011. Communities of ants (Hymenoptera Formicidae) in different Atlantic rain forest phytophysionomies. Sociobiology 58(3): 779-799.
- Ribas C. R., R. R. C. Solar, R. B. F. Campos, F. A. Schmidt, C. L. Valentim, and J. H. Schoereder. 2012. Can ants be used as indicators of environmental impacts caused by arsenic? Insect Conserv 16: 413421.
- Ryder Wilkie K. T., A L. Mertl, J. F. A. Traniello. 2010. Diversity of ground-dwelling ants (Hymenoptera: Formicidae) in primary and secondary forests in Amazonian Ecuador. Myrmecological News(12): 139-147
- Santoandre S., J. Filloy, G. A. Zurita, and M. I. Bellocq. 2019. Ant taxonomic and functional diversity show differential response to plantation age in two contrasting biomes. Forest Ecology and Management 437: 304-313.
- Santos P. P., A. Vasconcelos, B. Jahyny, and J. H. C. Delabie. 2010. Ant fauna (Hymenoptera, Formicidae) associated to arboreal nests of Nasutitermes spp. (Isoptera, Termitidae) in a cacao plantation in southeastern Bahia, Brazil. Revista Brasileira de Entomologia 54(3): 450-454.
- Santschi F. 1925. Fourmis des provinces argentines de Santa Fe, Catamarca, Santa Cruz, Córdoba et Los Andes. Comunicaciones del Museo Nacional de Historia Natural "Bernardino Rivadavia" 2: 149-168.
- Santschi F. 1939. Résultats scientifiques des croisières du navire-école belge, "Mercator". XIV. Formicidae s. lt. Mémoires du Musée Royal d'Histoire Naturelle de Belgique. (2)15: 159-167.
- Siqueira de Castro F., A. B. Gontijo, P. de Tarso Amorim Castro, and S. Pontes Ribeiro. 2012. Annual and Seasonal Changes in the Structure of Litter-Dwelling Ant Assemblages (Hymenoptera: Formicidae) in Atlantic Semideciduous Forests. Psyche doi:10.1155/2012/959715
- Siqueira de Castro F., A. B. Gontijo, W. Duarte da Rocha, and S. Pontes Ribeiro. 2011. As comunidades de formigas de serapilheira nas florestas semidecíduas do Parque Estadual do Rio Doce, Minas Gerais. MG.BIOTA, Belo Horizonte 3(5): 5-24.
- Smith M. R. 1937. The ants of Puerto Rico. Journal of Agriculture of the University of Puerto Rico 20: 819-875.
- Sobrinho T., J. H. Schoereder, C. F. Sperber, and M. S. Madureira. 2003. Does fragmentation alter species composition in ant communities (Hymenoptera: Formicidae)? Sociobiology 42(2): 329-342.
- Solomon, S.E. and A.S. Mikheyev. 2005. The ant (Hymenoptera: Formicidae) fauna of Cocos Island, Costa Rica. Florida Entomologist 88(4):415-423
- Ulyssea M. A., and C. R. F. Brandao. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia 57(2): 217224.
- Ulysséa M. A., C. R. F. Brandão. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia 57(2): 217-224.
- Vargas R. P. 1964. Clave para identificar los Formicidae de la provincia de Chiclayo. Revista Peruana de Entomologia 7(1): 98-102.
- Vasconcelos, H.L. and J.M.S. Vilhena. 2006. Species turnover and vertical partitioning of ant assemblages in the Brazilian Amazon: A comparison of forests and savannas. Biotropica 38(1):100-106.
- Vasconcelos, H.L., J.M.S. Vilhena, W.E. Magnusson and A.L.K.M. Albernaz. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. Journal of Biogeography 33:1348-1356
- Viana-Silva F. E. C., and C. M. Jacobi. 2012. Myrmecofauna of Ironstone Outcrops: Composition and Diversity. Neotrop Entomol 41: 263271.
- Vittar, F. 2008. Hormigas (Hymenoptera: Formicidae) de la Mesopotamia Argentina. INSUGEO Miscelania 17(2):447-466
- Vittar, F., and F. Cuezzo. "Hormigas (Hymenoptera: Formicidae) de la provincia de Santa Fe, Argentina." Revista de la Sociedad Entomológica Argentina (versión On-line ISSN 1851-7471) 67, no. 1-2 (2008).
- Weber N. A. 1938. The food of the giant toad, Bufo marinus (L.), in Trinidad and British Guiana with special reference to the ants. Annals of the Entomological Society of America 31: 499-503.
- Weber N. A. 1944. The tree ants (Dendromyrmex) of South and Central America. Ecology 25: 117-120.
- Wheeler W. M. 1905. The ants of the Bahamas, with a list of the known West Indian species. Bulletin of the American Museum of Natural History 21: 79-135.
- Wheeler W. M. 1908. The ants of Jamaica. Bulletin of the American Museum of Natural History 24: 159-163.
- Wheeler W. M. 1908. The ants of Porto Rico and the Virgin Islands. Bulletin of the American Museum of Natural History 24: 117-158.
- Wheeler W. M. 1916. Ants collected in British Guiana by the expedition of the American Museum of Natural History during 1911. Bulletin of the American Museum of Natural History 35: 1-14.
- Wheeler W. M. 1919. The ants of Tobago Island. Psyche (Cambridge) 26: 113.
- Wheeler W. M. 1922. The ants of Trinidad. American Museum Novitates 45: 1-16.
- Wheeler W. M. 1927. Chinese ants collected by Professor S. F. Light and Professor N. Gist Gee. American Museum Novitates 255: 1-12.
- Wheeler W. M., and W. M. Mann. 1914. The ants of Haiti. Bulletin of the American Museum of Natural History 33: 1-61.
- Wheeler, William Morton. 1911. Additions to the Ant-Fauna of Jamaica. Bulletin American Museum of Natural History. 30:21-29.
- Wheeler, William Morton. 1911. Ants Collected in Grenada, W.I. by Mr. C. T. Brues. Bulletin of the Museum of Comparitive Zoology at Harvard College. 54(5):166-172.
- Wheeler, William Morton. 1916. Ants Collected in Trinidad by Professor Roland Thaxter, Mr. F. W. Urich, and Others. Bulletin of the Museum of Comparitive Zoology at Harvard University. 40(8):322-330
- Wheeler, William Morton. 1923. Report on the Ants. The University of Iowa Studies in Natural History. 10(3):3-9.
- Wild, A. L. "A catalogue of the ants of Paraguay (Hymenoptera: Formicidae)." Zootaxa 1622 (2007): 1-55.
- Zhang W., and S. Zhou. 2016. An investigation on Formicidae species of Nanling National Park. Journal of Huizhou University 36(3): 27-30.
- Zhao S., F. L. Jia, G. Q. Liang, Y. L. Ke, W. J. Tian. 2009. Ants and their distribution in Guangdong Province, China. Journal of Environmental Entomology 31(2): 156-161.
- da Silva de Oliveira A. B., and F. A. Schmidt. 2019. Ant assemblages of Brazil nut trees Bertholletia excelsa in forest and pasture habitats in the Southwestern Brazilian Amazon. Biodiversity and Conservation 28(2): 329-344.
- do Nascimento, I.C. 2006. Fenologia dos Voos de Acasalamento em Formigas Tropicais
- dos Santos Bastos A. H. , and A. Y. Harada. 2011. Leaf-litter amount as a factor in the structure of a ponerine ants community (Hymenoptera, Formicidae, Ponerinae) in an eastern Amazonian rainforest, Brazil
- Tandem running
- Invasive
- Tropical
- South subtropical
- South temperate
- Eucharitid wasp Associate
- Host of Chalcura deprivata
- Host of Kapala sp.
- Host of Kapala terminalis
- Host of Schizaspidia convergens
- Phorid fly Associate
- Host of Apocephalus lopesi
- Host of Dohrniphora longirostrata
- Host of Dohrniphora sp.
- Host of Dohrniphora sp. (longi-gp females
- Nematode Associate
- Host of Mermithidae (unspecified "Mermix")
- Karyotype
- Species
- Extant species
- Formicidae
- Ponerinae
- Ponerini
- Odontomachus
- Odontomachus haematodus
- Ponerinae species
- Ponerini species
- Odontomachus species
- Need Overview
- Need Body Text

