Mycetagroicus inflatus
| Mycetagroicus inflatus | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Mycetagroicus |
| Species: | M. inflatus |
| Binomial name | |
| Mycetagroicus inflatus Brandão & Mayhé-Nunes, 2008 | |
According to R. Feitosa field book (our translation from Portuguese) “the ants were collected at midafternoon of a cloudy day. In spite of the heavy rains of the preceding days (onset of the rainy season) the river water level was relatively low; leaving exposed a wide sand strip, with scattered bushes. The banks in this particular place are covered by gallery forest. The ants were found moving slowly towards their nest opening, which consisted of a single rounded minute opening in the sand, without any mound or crater. We waited for some minutes for other ants to come out from the nest, but they did not show up. Close to this nest, we found a nest of Mycetophylax emeryi (=Kalathomyrmex emeryi) and of an unidentified Dorymyrmex.” (Brandão & Mayhé-Nunes 2008)
Identification
Brandão & Mayhé-Nunes (2008) - Mycetagroicus inflatus has the triangular shape of the clypeal minute projections similar to those of Mycetagroicus triangularis, but can be distinguished from it by the frontal carinae not constricted above the frontal lobes, and by the peculiar relatively shorter pilosity. Furthermore, M. inflatus differs from all other species of the genus by the comparatively more prominent posterior corners of the head; the deeper notch on the vertexal margin; the reticulated integument covered with curved, short hairs; mandibles with six teeth; anterior clypeal margin without median notch; weakly impressed, triangular frontal area; conical and blunt pronotal median and propodeal projections; additional minute pair of projections on mesonotum after the anterior tumuli; deeper impression on the posterior region of postpetiole dorsum; and absence of a keel on the sternum I of gaster.
Jesovnik et al. (2013) - Queen The gyne of M. inflatus is, as in many ant species, quite similar to the worker except for modifications typical for the caste such as the presence of ocelli, the morphology of the mesosoma associated with wings, and the slightly larger size. In addition, in the worker the eyes are smaller, the frontoclypeal teeth are less pronounced, the frontal lobes are narrower, median anterior tubercles are present on the pronotum, and the petiole is shorter. The gyne of M. inflatus can be distinguished from the gynes of other closely related attine genera by characters that are synapomorphic for the genera. Sericomyrmex gynes, for instance, are larger in size and covered with dense pilosity, have cordate heads, have gasters that are laterally straight instead of rounded, and have frontal lobes that are more developed than those in Mycetagroicus. Gynes of Mycetomoellerius, Paratrachymyrmex and Trachymyrmex species are in most cases larger in size and have integuments that are moderately to strongly tuberculate, frequently appearing spiny, most noticeably on the posterior lobes of head and on the gaster, which in M. inflatus are smooth. Cyphomyrmex gynes can be similar in size to gynes of M. inflatus, but are immediately distinguished by the strongly expanded frontal lobes, often covering a large portion of the head, and in the strigatus and wheeleri groups accompanied by deep antennal scrobes. The gyne of the congeneric Mycetagroicus triangularis can be distinguished from that of M. inflatus by its slightly larger size, the presence of a median longitudinal ridge posterior to the frontal triangle, the presence of a distinct parapsidal furrow, the presence of scutellar processes, and the rugulose microsculpture on the dorsum of first gastral segment (A4), which also bears two pronounced ridges laterally.
Males of M. inflatus have 12-segmented antennae, a deviation from the usual 13-segmented antennae present in the males of most attine species, although the 12-segmented condition has arisen independently elsewhere in the tribe in the genus Sericomyrmex, in some Mycetophylax species (e.g., Mycetophylax faunulus and Mycetophylax auritus), in Mycetomoellerius opulentus, and in a number of social parasites in the genus Acromyrmex (Gallardo, 1916). Reductions in male antennal segment number are even more dramatic in males of the socially parasitic attine species Mycocepurus castrator (Rabeling and Bacci, 2010) and Pseudoatta argentina (Gallardo, 1916; Schultz et al., 1998), which have 11-segmented antennae. The male of M. inflatus can be distinguished from males of closely related genera by the following combination of characters: presence of 12 antennal segments, reticulate-punctuate integument (even on the clypeus), dark brown color, transversely costate groove on the mesopleuron, and propodeal teeth very reduced. Males of Sericomyrmex can be distinguished from males of M. inflatus by the presence of dense appressed pilosity covering the whole body and longer, thicker, decumbent to erect hairs on the head, mesosoma, and dorsum of the gaster. Males of Mycetomoellerius, Paratrachymyrmex and Trachymyrmex usually also have denser pilosity, are sometimes covered with spiny tubercules on the head and gaster, and usually have sharp propodeal spines and humeral tubercles. Males of the closely related species Cyphomyrmex costatus and Cyphomyrmex wheeleri have 13 antennal segments, strongly developed frontal lobes, antennal scrobes that reach the posterior border of the head, and sharp propodeal spines.
Keys including this Species
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: -8.75271° to -8.80157°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
|
Jesovnik et al. (2013) investigated a few aspects of the biology of M. inflatus.
Field work was conducted from 5 to 10 October 2012 in eastern Para State, Brazil, at the end of the dry season. During this time period the first rains of the rainy season occurred. All nests were found at two localities on the west coast of the Araguaia River, a southern component of the Amazon River Basin, across from the town of Araguacema, Tocantins, in an area characterized as ‘‘dense alluvial forest’’ (Silva, 2007). At ‘‘Locality 1’’ (S 08.75387°, W 49.54544°, elevation 164 m), nest entrances occurred approximately 15 m from the river’s edge in an area about 7 m above water level, which was at its seasonal low point; at ‘‘Locality 2’’ (S 08.80157°, W 49.57368°, elevation 176 m), they occurred approximately 5 m away from the river’s edge and about 5 m above the water level. At both localities the beach area is sandy, with occasional low bushes, and banks are covered with gallery forest. Based on information from local inhabitants, both Locality 1 and Locality 2 are entirely submerged during the rainy season (December–March).
In both localities, nests were located in the morning by following foraging workers returning with bait (Cream of Rice cereal) to their nest entrances. The nests were then carefully excavated.
Nest architecture
Four nests were excavated. All nests had a single, simple, very inconspicuous entrance hole approximately 2–3 mm in diameter. In no case was the nest entrance surrounded by a mound of excavated soil particles, differing in this regard from entrances of the congeneric Mycetagroicus cerradensis (Solomon et al., 2011). Nests contained 2–4 chambers, which varied from 2–8 cm in width. The deepest chamber encountered was 310 cm deep and contained garden and ants, but no queen. The shallowest chamber encountered was 22 cm deep and contained no fungus or ants, only loose sand. The shallowest chamber that contained ants was 68 cm deep, and the shallowest chamber that contained fungus garden was 75 cm deep. Tunnels connecting chambers were observed in some cases and were very straight and perpendicular to the surface (Fig. 1). The walls of two nest chambers, from nests TRS121009-01 and -02, were punctuated with multiple holes (Fig. 1c). Colony sizes ranged from approximately 30–100 workers.
All four excavated nests of Mycetagroicus inflatus had similar characteristics. The walls of one chamber in each of the two nests from Locality 2 (TRS121009-01: chamber depth not recorded; TRS121009-02: chamber 75 cm deep) were punctured by numerous holes of unknown purpose. It is possible that such punctures were present in additional chambers but were overlooked. It is highly unlikely that all of the holes were the openings of tunnels because no ants were observed entering or exiting and, if they were tunnel openings, they would connect with dozens of horizontal tunnels whereas no such tunnels were encountered; all of the tunnels observed during excavation were vertically arranged. One possibility is that the holes are somehow associated with flooding, perhaps serving as shelters for the ants during the months that the nests are submerged or serving in some way to capture and hold pockets of air. This conjecture gains support from the observation that similarly punctured chamber walls, as described above, were observed in the deepest chambers of colonies of the sympatric but very distantly related species Kalathomyrmex emeryi, colonies of which are certainly flooded during the rainy season. In addition to being punctured by numerous holes, unlike those of M. inflatus the chamber walls of K. emeryi were lined with a layer of brown clay.
The nest architecture of Mycetagroicus cerradensis, the only other congeneric species for which nesting biology is known, shares some characteristics with that of M. inflatus, including: a single nest entrance, chambers that are more or less vertically arranged below the nest entrance, and an extremely deep lowermost garden chamber (Solomon et al., 2011). Solomon et al. (2011) suggested that M. cerradensis moves its fungus garden seasonally, using deeper chambers in the dry season and shallower chambers in the wet season. This hypothesis applies equally to M. inflatus because, in both species, nests were excavated in the dry season and the uppermost nest chambers were found to be empty. Seasonal repositioning of fungus gardens has been recorded for some other fungus-growing ants, including two species of the genus Mycocepurus (Rabeling et al., 2007) and in the North American species Atta texana and Acromyrmex versicolor (Moser, 1962, 2006; Mueller et al., 2011a, b).
Fungal symbiont
ITS sequences indicate that the fungal cultivars collected from three nests of M. inflatus (JSC121006-02, TRS121006-07, and TRS121007-01) all belong to subclade F of the socalled ‘‘Clade 2’’ of lower attine (G3) leucocoprineaceous cultivars (Mehdiabadi et al., 2012; Mueller et al., 1998). Subclade F is arguably a single fungal species that is also associated with Mycetophylax faunulus, Mycocepurus smithii, Myrmicocrypta cf. buenzlii, and other attine species from Ecuador, Trinidad, Guyana, and Brazil (Mehdiabadi et al., 2012; unpubl. data). Remarkably, the fungal species cultivated by M. inflatus in Para is also grown by its congener Mycetagroicus cerradensis (Solomon et al., 2011, accession number in GenBank HM245775) over 1,000 km to the south in Minas Gerais.
Perhaps the most potentially important result of this study is the identification of the fungal cultivars grown by M. inflatus at the study sites on the Araguaia River. The gardens from all three collected nests belong to the same fungal species, suggesting that M. inflatus may depart from most lower attine species in possessing a high degree of symbiont fidelity. Even more surprising, M. inflatus cultivates the same fungal species as its congener M. cerradensis in Minas Gerais, Brazil, over 1000 km to the south (Solomon et al., 2011).
Arthropod symbiont
While following a M. inflatus forager carrying Cream of Rice bait back to its nest, a fly, later identified as belonging to the genus Pholeomyia sp. (Milichiidae: Diptera), was observed following the worker by walking a short distance behind it (JSC, pers. obs.). Sabrosky (1959) reported similar observations by W. L. Brown and E. O. Wilson of a Pholeomyia fly following a worker of the fungus-growing ant Trachymyrmex septentrionalis McCook in Florida and suggested a possible association of certain Pholeomyia species with fungus-growing ants. Later, Waller (1980) reported finding Pholeomyia texensis Sabrosky entering the nests of Atta texana by riding on the cut leaves, where the fly larvae presumably feed on nest refuse. Larvae of some species of milichiids have close associations with Hymenoptera (Sabrosky, 1959; Krombein, 1967; Moser and Neff 1971; Melo, 1996; Wild and Brake, 2009; Swann, 2010).
Castes
Worker

| |
| . | |
Queen
Images from AntWeb
    
| |
| Queen (alate/dealate). Specimen code antweb1008121. Photographer Ana Jesovnik, uploaded by California Academy of Sciences. | Owned by USNM, Washington, DC, USA. |
Male
Images from AntWeb
   
| |
| Male (alate). Specimen code antweb1008120. Photographer Ana Jesovnik, uploaded by California Academy of Sciences. | Owned by USNM, Washington, DC, USA. |
Larva
Images from AntWeb
  
| |
| Other (larva/pupa). Specimen code antweb1008122. Photographer Ana Jesovnik, uploaded by California Academy of Sciences. | Owned by USNM, Washington, DC, USA. |
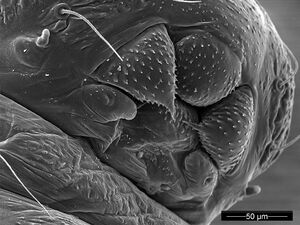
| |
| Other (larva/pupa). Specimen code antweb1008123. Photographer Ana Jesovnik, uploaded by California Academy of Sciences. | Owned by USNM, Washington, DC, USA. |
Phylogeny
| Mycetagroicus |
| ||||||||||||
Based on Micolino et al., 2020 (note Mycetagroicus urbanus not included).
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- inflatus. Mycetagroicus inflatus Brandão & Mayhé-Nunes, 2008: 349, figs. 1-4 (w.) BRAZIL.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
(holotype-paratype). TL 2.61-2.64; HL 0.75-0.76; HW 0.71-0.72; IFW 0.35-0.35; ScL (only for holotype) 0.65; HWL 0.46-0.44; MeL 0.94-0.93; PLL 0.13-0.17;. PPL 0.34-0.37; GLL 0.75-0.73; HfL 0.85-0.84.
Brown-yellowish, with the head’s frontal region, tergum I and sternum I of gaster darker; the dorsum of the head being darker than all other body parts. Integument finely reticulated throughout the body. Scattered short hairs all over the body and appendages, slightly curved at tip; longer ones confined to hypostoma, median region of clypeus, and on mandibles, in special on the mandibular apexes. Head in full face view a little longer than broad (CI 95). Outer border of mandible straight; masticatory margin with apical, subapical and 4 triangular teeth, gradually diminishing in size towards base. Clypeus divided transversally by a ridge (better seen under the SEM; see also Fig.4), from where the clypeal setae arise; median seta distinct from anteclypeal seta; latero-median area of clypeus with a sharp triangular tooth at each side. Frontal area shallowly impressed. Frontal lobe semicircular, moderately approximate (FLI 49), with smooth free border. Frontal carina ending just after the expansion of the frontal lobes, parallel. Preocular carinae conspicuous, straight in front of the eyes, curving obliquely towards the head´s median line, ending a little after the level of the frontal carinae ends. Vertex margin in full-face view strongly notched in the middle; the head posterior corners inflated. Inferior corner of occiput, in side view, more or less angular, emmarginate. Eye bulging, surpassing the lateral border of the head, with about 11 facets across greatest diameter. Antennal scape projecting beyond the tip of the head’s posterolateral corner by a distance which exceeds its maximum width; the distal three fourths of scape gently incrassate. Only funicular segments I and VIII-X longer than broad, the others sub-equal in size and width.
Mesosoma. Dorsal and lateral faces of pronotum not separated by a carina, emmarginate, with low and blunt triangular projections at the meeting of these faces; anteroinferior corner of pronotum with a rounded tooth; inferior margin smooth; paired median pronotal teeth separate, conical and blunt. Mesonotum with two very low, wide, flat semicircular tumuli anteriorly, divided by a depression, followed by a widely spaced pair of minute triangular projections and then by a more approximate, not so low, pair of blunt projections; posterior margin oblique in lateral view. Metanotum indistinct, not constricted in dorsal view. Basal face of propodeum emmarginate, with 2 or 3 low rounded unconnected projections; triangular propodeal spine very low, truncate and blunt. Hind femora shorter than mesosoma length.
Waist and gaster. Dorsum of petiolar node without ridges, ascending in lateral view towards two spaced faint projections, bearing each a pit and its hair; petiole broader than long in dorsal view. Postpetiole as long as broad in dorsal view, dorsal and posterior margins straight, in lateral and dorsal view, respectively; dorsally with a swallow impression, near the posterior margin. Gaster, when seen from above, rather suboval, posteriorly rounded. Tergum I without longitudinal keel or furrow. Sternum I without a sagital keel.
Queen
Jesovnik et al. (2013) - HW 0.60–0.72, HL 0.72–0.76, ScL 0.62–0.66, IFW 0.38–0.39, ML 0.20–0.26, EL 0.15–0.17, WL 1.0–1.1, HfL 0.79–0.87, PL 0.18–0.23, PPL 0.32–0.37, GL 0.89–1.14, CI 84–94, FLI 55–62, SI 91–102 (n = 7).
Posterolateral corners of head, mesosoma, petiole, and postpetiole light ferruginous brown; gaster dark ferruginous brown. Entire dorsum of head in full-face view fuscous, darker than gaster. Mandibles, antennae, and anterior borders of frontal lobes distinctly lighter than the rest of the dorsum of the head. Integument finely reticulate-punctuate, opaque. Erect hairs absent, most of the body covered with short, white, appressed hairs.
Head. In full-face view slightly longer than broad (CI 84–94), posterior corners of cephalic margin rounded, slightly notched medially. Mandibles triangular, dorsally striate, masticatory (inner) margin of mandible with 5 teeth, the distal-most tooth noticeably prolonged and larger than the rest. Outer margin of mandible straight. Margin of anterior clypeal apron convex, with a very broad and shallow but distinct median notch. Median clypeal seta (~0.15 mm) arising from border of clypeal apron and clypeal anterior margin, flanked on either side by a pair of additional, long setae as well as 2–3 shorter setae. Distinct frontoclypeal teeth present (‘‘triangular lateral tooth’’ of Brandao and Mayhe-Nunes, 2008), best seen in lateral view. Frontal lobes evenly rounded, separated by a fingerlike extension of the clypeus extending posterad to the level of the eyes. Frontal carinae short, diverging toward corners of the head, not extending beyond median ocellus. Preocular carina distinct, extending posterad from mandibular insertion, then curving medially above eye level. Area laterad of frontal lobes, the ill-defined antennal scrobe, devoid of hairs. Eyes convex, 13 ommatidia across largest diameter of dorso-ventral axis and 10 ommatidia across smallest diameter. Three ocelli present, all similar in size. Antennal scape extending only slightly (~0.02 mm) past posterior border of head. Scape noticeably curved ventrad in basal one third, best seen in posterior view with scape positioned at 90° from median axis. Antenna 11-segmented, lacking distinctive antennal club. First funicular segment and segments 8–10 longer than broad, segments in between almost subquadrate. Palp formula 4, 2.
Mesosoma. Lateral pronotal tubercle present, dentiform. Anterior pronotal tubercle absent (‘‘paired median pronotal teeth’’ of Brandao and Mayhe-Nunes, 2008). Antero-inferior corner of pronotum almost forming a right angle, best seen in lateral view. Anapleural suture present (‘‘median episternal groove’’ of Snodgrass, 1910). Scutum lacking notauli or other sutures, shallowly impressed longitudinally. Parapsidal furrow absent. Axillae (‘‘anterior division of scutellum’’ sensu Snodgrass, 1910; ‘‘prescutellum’’ sensu Tulloch, 1935; ‘‘paraptera’’ sensu Brandao and Mayhe-Nunes, 2008) relatively large, laterally rounded and medially constricted. Scutellum mostly flat in profile view, weakly concave and narrowing posteriorly in dorsal view, posterior border medially emarginate, not forming scutellar processes. Propodeal teeth short, blunt, and directed posterolaterally. Propodeal carinae absent. Propodeal spiracle directed posterad, mounted on a tumulus, best seen in dorsal view. Propodeal lobe lamellate, connected by a short, longitudinal carina, along bulla of metathoracic gland, to the dorsum of the propodeal spiracle, best seen in dorsolateral view.
Wings. Transparent, covered with minute pilosity, veins brown. Forewing (length: 3.62 mm) with 5 closed cells (terminology follows Goulet and Huber, 1993): costal (C), radial (R), cubital (Cu), first radial 1 (1R1), and first radial 2 (2R1). Pterostigma not visible. Hindwing (length: 2.57 mm) with reduced venation, just one closed cell, and 6 hamuli.
Metasoma. Petiole compact, relatively small, petiolar peduncle vestigial. Subpetiolar process present anteriorly, pointing forward, sometimes not clearly visible because concealed by propodeal lobes. From ventral view clearly visible as a spine. Petiole dorsum with two small denticles near its posterior border. Postpetiole large, >1.59 the width of the petiole, slightly broader than long in dorsal view. Posterior border of postpetiole wider than the rest of petiole, bearing protruding transverse plate that is rounded laterally and slightly emarginated medially (Fig. 2e). In dorsal view, gaster elliptical, narrowing posteriorly, lacking ridges or noticeable sculpture, but having finely punctuated microsculpture. Rows of long, suberect hairs present on posterior borders of gastral sternites 2–4 (A5–A7). Gastral tergite and sternite I subequal in length, the posteriormost tip of the gaster formed by segment A7 in contrast to the (presumably derived) condition in some Attini, in which gastral tergite I longer than sternite I so that posteriorly it overlaps the remaining segments, which are shifted anterad.
Male
Jesovnik et al. (2013) - HWm 0.53–0.61, IOD 0.37–0.40, HL 0.45–0.56, ScL 0.44–0.62, IFW0.15 0.19, ML 0.14–0.21, EL 0.13–0.19, WL 0.90–1.16, HfL 1.05–1.35, PL 0.14–0.20, PPL 0.21–0.29, GL 0.80–1.07, CI 77–81, FLI 41–44, SI 120–142 (n = 7).
Body color dark brown, integument reticulate-punctuate on head and mesosoma, gaster opaque with reticulate microsculpture. Erect hairs absent, short appressed white hairs covering head, dorsal side of mesosoma, gaster, and legs; only a few hairs present on lateral side of mesosoma.
Head. In full-face view longer than broad, posterior border smoothly rounded. Mandibles triangular, dorsally striate, basal angle rounded, masticatory margin with three teeth, all arising in the distal half, an unusual arrangement shared with males of Mycetomoellerius urichii and perhaps other Mycetomoellerius species. Clypeus evenly reticulate, without frontoclypeal carinae or teeth as seen in worker and queen. Anterior margin of clypeal apron convex, with shallow median notch. Median clypeal seta (~0.12 mm) arising at the point where posterior margin of clypeal apron and anterior clypeal margin meet flanked by one much shorter seta on each side. Frontal lobes small, short, not completely covering the antennal condyle, broadly separated by a fingerlike extension of the clypeus extending posterad. Frontal carinae short, extending to the level of the posterior border of the eyes. Preocular carina distinct, curving posteriorly toward the midline. Eyes convex, large, 16 ommatidia across largest diameter of dorso-ventral axis, 15 ommatidia across the smallest diameter of the eye. Three similarly sized ocelli present. Antennal scape straight, long, extending well beyond the occiput. Antenna 12-segmented, a departure from the plesiomorphic number of 13 for ant males (including attine males), the first funicular segment (pedicel) as long as second funicular segment, or slightly longer. First funicular segment longer than broad and thicker than the second funicular segment, but not thicker than the most distal segments. Palp formula 4, 2.
Mesosoma. Lateral pronotal tubercles absent. Anteroinferior corner of pronotum rounded. Anapleural suture present, dividing mesopleuron into katepisternum and anepisternum. A wide, transversely costate groove present on dorsoposterior border of anepisternum below insertion of wing. In some males the anapleural suture can also be transversely costate. Scutum with shallow, complete, V-shaped notauli, dividing the scutum into an anteromedian area (‘‘prescutum’’) and two lateral areas. Median mesoscutal sulcus present, fading posteriorly. Parapsidal lines present. Axillae relatively large, rounded laterally, constricted medially. Scutellum slightly inflated, narrowing posteriorly, posterior border with two short, blunt denticles. Propodeal teeth reduced to short, blunt denticles.
Wings. Forewing (length: 3.81 mm), hindwing (length: 2.7 mm). Venation and appearance same as in female, except in male spur of cross vein 1 m-cu is visible protruding from the bottom of 1R1 cell and 7 hamuli instead of 6 on hind wing.
Metasoma. Petiole compact, node in dorsal view rounded, slightly broader than long. Petiolar sternite narrowing to a forward directed, sharp keel. Postpetiole in dorsal view rounded, slightly broader than long, posteriorly emarginate. Integument of tergum 1 of gaster (i.e., A4) more shining than the other body parts, but still subopaque due to the finely reticulate microsculpture; bearing sparse, white, appressed hairs. In dorsal view, gaster elliptical, narrowing posteriorly, without any carinae or other macrosculpture. Gastral tergite and sternite I subequal in length and gastral tergites 2–5 (i.e., A5–A8) visible dorsally or posterodorsally. Rows of long, suberect hairs present on posterior borders of gastral sternites 2–5 (A5–A8).
Larva
Jesovnik et al. (2013) - Specimens examined: three last-instar worker larvae and two worker prepupae (i.e., post-feeding last instars), all from nest collection TRS121006-07. Profile ‘‘attoid’’ sensu Wheeler and Wheeler 1976, i.e., with a moderately curved, ventrally shortened profile. As in all other Attini, thoracicabdominal articulation absent, thoracic intersegmental constrictions superficial, deep lateral depressions associated with abdominal spiracles absent, and leg vestiges present as open slits. As in most other Attini, dorsal and lateral body surfaces devoid of setae. Differing from other Attini in the remarkably small number of head and ventral setae. Head devoid of setae except for two setae on each gena, a state previously unknown in attine larvae; ventral thoracic segments 1–3 each with a single pair of setae laterally; and two short setae on abdominal segment 10 ventral to the anus. Ventral thoracic segments 1 and 2 medially bearing multidentate spinules, thoracic segment 2 additionally with a low ventromedian boss, in combination with the genal and thoracic setae clearly functioning as a food anchor. As in most Neoattini, genal lobes present. Labrum monolobate, narrow, bulging, a synapomorphy for the Attini; anterior labral setae reduced to papillae. Mandibles typically attine: short, fleshy, subconical. A distinct, undivided apical mandibular tooth and no subapical teeth; spinules sparsely but evenly distributed on all mandibular surfaces. Mandibular gnathobases absent. Basal portions of maxillae fused with head capsule. As in all other Neoattini, maxillary palp widely removed laterad from galea. Galea reduced, present as two sensilla surmounting a low welt; a shallow pit distal and immediately adjacent to the galea. Maxillary palp digitiform, maxillary accessory palpal sensillum absent. One seta on each maxilla between the galea and palp, a second seta present laterad of the palp. As in most attines, labium feebly protruding and lateral sericteral protuberances absent; labial palps papilliform. Labial spinules present basad and absent distad of the sericteries. Hypopharyngeal spinules largely unidentate and moderately to sparsely distributed.
Type Material
Holotype and paratype workers: BRAZIL, Pará State: Santa Maria das Barreiras, Rio Araguaia, 30.ix.2005, R. R. Silva & R. Feitosa cols (deposited in Museu de Zoologia da Universidade de São Paulo). The workers were collected manually while foraging outside the nest, in a beach along the West bank of the Araguaia river, part of the Amazon River drainage.
Etymology
The name of this species refers to the inflated head posterior corners that differentiate this species from all other known Mycetagroicus.
References
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Brandão, C. R. F. and A. J. Mayhé-Nunes. 2008. A new species of the fungus-farming ant genus Mycetagroicus Brandão & Mayhé-Nunes (Hymenoptera, Formicidae, Attini). Revista Brasileira de Entomologia. 52(3):349-352.
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Cantone S. 2018. Winged Ants, The queen. Dichotomous key to genera of winged female ants in the World. The Wings of Ants: morphological and systematic relationships (self-published).
- Jesovnik, A. J. Sosa-Calvo & C.T. Lopes. 2013. Nest architecture, fungus gardens, queen, males and larvae of the fungus-growing ant Mycetagroicus inflatus Brandao & Maye-Nunes. Insectes Sociaux. 1-12.
- Moleiro, H.R., Da Silva-Melo, A., Giannotti, E. 2021. Nest architecture and animals associated with Neoponera verenae (Forel) (Formicidae, Ponerinae). Sociobiology 68, e6246 (doi:10.13102/sociobiology.v68i3.6246).
- Solomon S.E., Lopes C.T., Mueller U.G., Rodrigues A., Sosa-Calvo J., Schultz T.R. and Vasconcelos H.L. 2011. Nesting biology and fungiculture of the fungus–growing ant, Mycetagroicus cerradensis: New light on the origin of higher attine agriculture. Journal of Insect Science. 11:1-12.
References based on Global Ant Biodiversity Informatics
- Solomon S. E., C. Rabeling, J. Sosa-Calvo, C. Lopes, A. Rodrigues, H. L. Vasconcelos, M. Bacci, U. G. Mueller, and T. R. Schultz. 2019. The molecular phylogenetics of Trachymyrmex Forel ants and their fungal cultivars provide insights into the origin and coevolutionary history of ‘higher-attine’ ant agriculture. Systematic Entomology 44: 939–956.


