Hypoponera opaciceps
| Hypoponera opaciceps | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Ponerinae |
| Tribe: | Ponerini |
| Genus: | Hypoponera |
| Species: | H. opaciceps |
| Binomial name | |
| Hypoponera opaciceps (Mayr, 1887) | |
| Subspecies | |
| |
| Synonyms | |
| |
| Common Name | |
|---|---|
| Kadofushi-nise-hari-ari | |
| Language: | Japanese |
A cryptobiotic species that forages in leaf mold. They are frequently found nesting in decaying wood. Winged and apterous forms occur in both queens and males (Japanese Ant Image Database).
| At a Glance | • Invasive • Diploid male |
Identification
The relatively coarse, subopaque body sculpture, the shape of the head and petiole are the main distinguishing features for this species (Kempf 1962).
The nearly parallel front and rear faces of the worker petiole (in lateral view) and non-shining integument, due to minute, dense granulation, distinguish this species. The male has very pale wing veins (almost transparent) and slightly elongate eyes. We have seen occasional ergatoid queens (or workers with large eyes).
Keys including this Species
Distribution
South Carolina through Florida, west to Colorado; Central and South America (as far south as Uruguay), West Indies, Southeast Asia and Polynesia (Smith 1979). Reported from Nevada by Wheeler and Wheeler (1986) and Arizona by Mackay and Mackay (2002). It was probably transported by commerce from the Neotropics to Pacific tropical areas (Wilson and Taylor 1967). It occurs in southern California in moist areas, such as the edges of irrigation ditches and low spots in watered lawns. Colonies have been found in the soil of potted plants in Florida, which is a center of the nursery trade. This species is also common along beaches in Florida; some of the early transportation of the species might have been in soil ballast obtained from beaches.
Latitudinal Distribution Pattern
Latitudinal Range: 25.68015° to -34.583333°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Australasian Region: New Caledonia.
Indo-Australian Region: American Samoa, Fiji, French Polynesia, Hawaii, Micronesia (Federated States of), New Guinea, Palau, Philippines, Samoa, Tonga.
Nearctic Region: United States.
Neotropical Region: Argentina, Bahamas, Barbados, Bermuda, Brazil (type locality), Costa Rica, Cuba, Dominican Republic, Ecuador, Galapagos Islands, Guatemala, Guyana, Haiti, Lesser Antilles, Mexico, Netherlands Antilles, Panama, Paraguay, Peru, Puerto Rico, Trinidad and Tobago, Uruguay.
Palaearctic Region: China, Japan.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
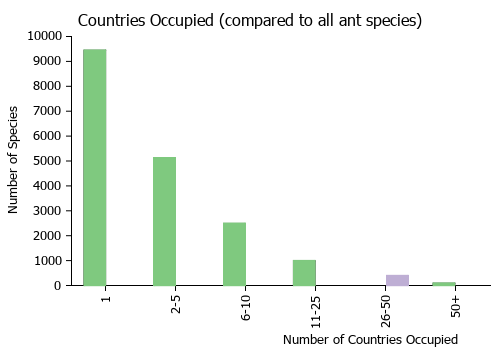
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Smith (1929) reports on a nest being taken from a partly decayed pine stump (Ocean Springs, MS). Deyrup, Davis and Cover (unpublished) noted this ant in Florida: "forms small colonies in open or partially shaded areas. Most common in wet or periodically flooded areas, where it tends to replace Hypoponera opacior in fallen logs, grass tussocks and accumulations of organic matter (see Van Pelt 1958, excerpt below). It sometimes occurs under piles of seaweed high up on the beach. This species is a rapid colonizer of disturbed areas such as lawns and ditches. In southern Florida alates may be found in flight in the afternoon and evening at any time of the year. Alate queens can deliver a noticeable sting when they become trapped under clothing or stuck to the skin by sweat.
Van Pelt (1958), reporting on this species within the Weleka Reserve in Florida: "Many of the nests of opaciceps, especially in marsh, contained one to several individuals which are evidently aberrant workers, These insects have large compound eyes, comparable to those of the queen, and the petiole is, more slender than that of the normal worker. It is perhaps significant that queens were not found in nests which contained these aberrant forms.
This species prefers the wet or flooded areas of the Reserve, and tends to replace P. trigona opacior (=Hypoponera opacior), a form which prefers high, dry areas. It has been taken abundantly in marsh; commonly in hydric hammock and river swamp; and rarely in Rutledge slash pine flatwoods, bayhead, and xeric hammock. In the Gainesville region, P. opaciceps was taken in longleaf pine flatwoods where there are more fallen logs than in the same plant association on the Reserve. It ought to occur also in at least the lower portions of mesic hammock.
Most often this ant nests in the bases of sawgrass plants between the appressed leaves. Many times the ant can be found in the wet or saturated moss-covered stumps of the plants where there is an intermingling of roots in the decomposing, appressed leaves and wet debris. The other nesting sites, in order of preference, are: 1) fallen logs, 2) dead stumps, 3) bases of living trees (under moss and litter at water surface), 4) in litter (wet), 5) palmetto roots on ground, 6) under mat of palmetto roots, and 7) under mat of palmetto trunks. In general, the nests are wet to saturated and built in debris. Most of the nests, especially those in sawgrass, are at the water surface, or just above or below it. (In this latter case, the tight growth of the plant parts seems to keep the water from the nest.) In situations which are less wet, the insect continues to simulate the above-mentioned nesting conditions in its choice of wet pulpy wood of logs, or the debris found under the bark of logs or stumps.
Of 5 nests counted, the number of workers varied from 15 to 84, with an average of 40 per nest. Each of these nests contained from one to three of the aberrant workers described above, and none contained a queen. Immature forms probably occur in all months, but from September through November very few were noticed in the nests. Females are produced from September to November, and males from October to November.
This is a fast moving and evasive ant which blends with the color of its surroundings. It is much less active in winter months, although this is the period winged forms are in the nest."
Regional Notes
New Mexico
Mackay and Mackay (2002) - Habitat. Cottonwood-willow forests, riparian habitats and sagebrush, urban areas. Biology. This ant nests under stones. The specimens in Albuquerque were collected on sandbars along the Rio Grande river in pitfall traps (Fagerlund, pers. comm.).
Life History Traits
- Queen type: winged or dealate; ergatoid
- Male type: winged; ergatoid
Castes
 
| |
| . | |
Worker
   
| |
| . | Owned by Museum of Comparative Zoology. |
Images from AntWeb
   
| |
| Worker. Specimen code casent0104122. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
   
| |
| Worker. Specimen code casent0104662. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by AMNH, New York, NY, USA. |
   
| |
| Worker. Specimen code casent0104663. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by AMNH, New York, NY, USA. |
   
| |
| Worker. Specimen code casent0173715. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ALWC, Alex L. Wild Collection. |
Images from AntWeb
    
| |
| Worker. Specimen code casent0173294. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CDRS, Galapagos, Ecuador. |
Queen
Images from AntWeb
    
| |
| Queen (alate/dealate). Specimen code casent0173292. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CDRS, Galapagos, Ecuador. |
Male
Images from AntWeb
    
| |
| Male (alate). Specimen code casent0103959. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ABS, Lake Placid, FL, USA. |
     
| |
| Male (alate). Specimen code casent0173293. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CDRS, Galapagos, Ecuador. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- opaciceps. Ponera opaciceps Mayr, 1887: 536 (w.q.) BRAZIL. Smith, M.R. 1929: 545 (m.); Wheeler, G.C. & Wheeler, J. 1964b: 453 (l.). Combination in Hypoponera: Taylor, 1967a: 11. Senior synonym of perkinsi (and its junior synonym andrei): Wilson & Taylor, 1967: 28; of postangustata: Wild, 2007b: 54. Current subspecies: nominal plus cubana, gaigei, jamaicensis, pampana,. See also: Smith, M.R. 1936: 428; Wheeler, W.M. 1937c: 59; Kempf, 1962b: 7.
- andrei. Ponera andrei Emery, 1900c: 318 (footnote), pl. 8, fig. 47 (w.) NEW CALEDONIA. Junior synonym of perkinsi: Wilson, 1958d: 334.
- perkinsi. Ponera perkinsi Forel, 1899a: 117 (w.q.m.) HAWAII. Senior synonym of andrei: Wilson, 1958d: 334. Junior synonym of opaciceps: Wilson & Taylor, 1967: 28. See also: Wheeler, W.M. 1909c: 271.
- postangustata. Ponera opaciceps var. postangustata Forel, 1908c: 343 (w.) PARAGUAY. Combination in Hypoponera: Kempf, 1972a: 123. Junior synonym of opaciceps: Wild, 2007b: 54.
Taxonomic Notes
This species has a number of subspecies, named during a period when ants from widely separated areas were often assumed (usually correctly) to be different species or subspecies. In this case, since H. opaciceps seems to be readily transported by humans, it is likely that most of these far-flung subspecies are either trivial, non-geographic variants, or different species.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Description
Worker
Smith (1936) - Length: 3.2-3.4 mm. Head, excluding mandibles, longer than broad, posterior border faintly emarginate, the sides convex, and the posterior angles rounded. Eyes small, with approximately 10-12 facets, situated at a distance less than twice their greatest diameter from the base of the mandibles. Mandibles with more irregular teeth than with Ponera pennsylvanica, the front teeth not only larger but more regular. Clypeus convex medianly. No frontal area. A faint frontal furrow extends half the distance or beyond from the frontal carinae to the posterior border of the head. Scape not attaining the posterior border of the head; the funiculus clavate, the last segment apparently shorter than the three preceding segments. Thorax with distinct promesonotal and meso-epinotal sutures, not so laterally compressed, especially about the epinotum as with P. pennsylvanica; epinotal declivity faintly concave, with bluntly marginate sides, meeting the base in a well rounded, obtuse angle. Petiole viewed in lateral profile thick antero-posteriorly, scarcely narrower dorsally, convex anteriorly, concave posteriorly; viewed posteriorly the superior border merges into the sides in well rounded angles; tooth below although rectangular, apparently lacking the spiracular opening in front, and the point posteriorly, as with P. pennsylvanica.
Head, thorax, petiole, and gaster very densely and finely punctate, subopaque; meso- and meta-pleurae finely and longitudinally striated; mandibles, and epinotal declivity rather smooth and shining.
Pubescence yellowish gray, dense and appressed, covering all parts of the body. Hairs grayish, very noticeable on the clypeus and gaster where they are longest and most abundant; usually found sparingly on other parts of the body.
Color varying from brown to black; mandibles, antennal funiculi, legs and tip of gaster lighter.
Queen
Smith (1936) - Length: 3.7-4 mm. So similar to the worker as to hardly warrant a brief description. Very slightly larger. Compound eyes placed at a distance from the mandibles less than their greatest diameter. Frontal furrow extending to the anterior ocellus. Petiole proportionally smaller and narrower anteroposteriorly on the dorsal border than with the worker. Wings very hyaline, with light yellowish veins and stigma, each with a submarginal and discoidal cell.
Smith (1936) - Ergataner. - Length of head .68-.76 mm.; length of thorax .935-.965 mm. Head, including mandibles, longer than broad; posterior border almost straight, and sides subparallel, thus giving the head a more rectangular appearance than with the worker. Mandibles moderately broad, triangular, edentate stubs. Clypeus strongly convex, protuberant. Antennae 13-segmented, gradually but not strongly enlarging distally; scapes short, subcylindrical, approximately equal in length to the second, third, and fourth segments of the funiculi taken together. Compound eyes small, almost circular, separated from the base of the mandibles by a space equivalent to approximately one and one-half times their greatest diameter. Thorax short, robust; viewed laterally the pro-mesonotal and meso-epinotal sutures are very distinct, especially on the dorsum; mesonotum strongly gibbous, clearly projecting above the general surfaces of the pronotum and epinotum. Between the mesonotum and epinotum the suture is represented by a very strong constriction, following which, the epinotum forms a rather long and gentle arch terminating at the petiole. The basal surface and declivity of the epinotum merge into each other so gradually that they are hardly distinguishable. Petiole large, robust, anterior and posterior faces convex, superior border rounded. Gaster similar to that of the worker, but bearing prominent genital appendages.
Color sordid yellow; margins of compound eyes and antennal cavities black, articulations of legs and sutures of thorax brown.
The above description is based on two specimens which were taken from a colony of Ponera opaciceps at Landon, Mississippi, by G. W. Haug on August 25, 1930. No other ergataners of this species have been seen or recorded.
Type Material
Reported by Smith (1936) - Province of Saint Catherine, Brazil (Mayr). Kempf (1962) states he examined 4 type specimens sent to him from the Naturhistorisches Museum Wien, Vienna.
Etymology
opacus - shady, dark, dim + ceps - head.
References
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Borowiec, L. 2014. Catalogue of ants of Europe, the Mediterranean Basin and adjacent regions (Hymenoptera: Formicidae). Genus (Wroclaw) 25(1-2): 1-340.
- Camargo, K.S. de. 2011. Composicao e diversidade de "Poneromorfas" (Hymenoptera, Formicidae) em duas fitofisionomias de cerrado e padroes de distribuicao de "Poneromorfas", Pseudomyrmecinae e Cephalotini (Myrmicinae) para o Brasil. Thesis, Universidade de Brasilia.
- Carroll, T.M. 2011. The ants of Indiana (Hymenoptera: Formicidae). M.S. thesis, Purdue University.
- Dash, S.T. 2011. A taxonomic revision of the New World Hypoponera Santschi, 1938 (Hymenoptera: Formicidae). Ph.D. thesis, University of Texas, El Paso.
- Dash, S.T., Mackay, W.P. 2019. Capitulo 18. Genero Hypoponera. Hormigas de Colombia.
- Davis, T. 2009. The ants of South Carolina (thesis, Clemson University).
- Deyrup, M.A., Carlin, N., Trager, J., Umphrey, G. 1988. A review of the ants of the Florida Keys. Florida Entomologist 71: 163-176.
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- General, D.E.M., Buenavente, P.A.C., Rodriguez, L.J.V. 2020. A preliminary survey of nocturnal ants, with novel modifications for collecting nocturnal arboreal ants. Halteres 11: 1-12 (doi:10.5281/ZENODO.3707151).
- Gochnour, B.M., Suiter, D.R., Booher, D. 2019. Ant (Hymenoptera: Formicidae) fauna of the Marine Port of Savannah, Garden City, Georgia (USA). Journal of Entomological Science 54, 417-429 (doi:10.18474/jes18-132).
- Herrera, H.W., Baert, L., Dekoninck, W., Causton, C.E., Sevilla, C.R., Pozo, P., Hendrickx, F. 2020. Distribution and habitat preferences of Galápagos ants (Hymenoptera: Formicidae). Belgian Journal of Entomology, 93: 1–60.
- Hill, J.G. 2015. Ants (Hymenoptera: Formicidae) of the Big Thicket Region of Texas. Midsouth Entomologist 8: 24-34.
- Ipser, R.M., Brinkman, M.A., Gardner, W.A., Peeler, H.B. 2004. A survey of ground-dwelling ants (Hymenoptera: Formicidae) in Georgia. Florida Entomologist 87: 253-260.
- Kempf, W. W. 1962b. Miscellaneous studies on neotropical ants. II. (Hymenoptera, Formicidae). Stud. Entomol. 5: 1-38 (page 7, see also)
- Kureck, I.M., Nicolai, B., Foitzik, S. 2013. Similar performance of diploid and haploid males in an ant species without inbreeding avoidance. Ethology 119: 360–367 (doi:10.1111/eth.12073).
- Lubertazzi, D. 2019. The ants of Hispaniola. Bulletin of the Museum of Comparative Zoology, 162(2), 59-210 (doi:10.3099/mcz-43.1).
- Mackay, W.P. & Mackay, E.E. 2002. The Ants of New Mexico: 400 pp. Edwin Mellen Press, Lewiston, N.Y.
- Mayr, G. 1887. Südamerikanische Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 37: 511-632 (page 536, worker, queen described)
- Rodrigues, M.S., Vilela, E.F., Azevedo, D.O., Hora, R.R. 2011. Multiple queens in founding colonies of the neotropical ant Pachycondyla striata Smith (Formicidae: Ponerinae). Neotropical Entomology 40, 293–299 (doi:10.1590/s1519-566x2011000300001).
- Rosas-Mejía, M., Guénard, B., Aguilar-Méndez, M. J., Ghilardi, A., Vásquez-Bolaños, M., Economo, E. P., Janda, M. 2021. Alien ants (Hymenoptera: Formicidae) in Mexico: the first database of records. Biological Invasions 23(6), 1669–1680 (doi:10.1007/s10530-020-02423-1).
- Smith, M. R. 1929c. Descriptions of five new North American ants, with biological notes. Ann. Entomol. Soc. Am. 22: 543-551 (page 545, male described)
- Smith, M. R. 1936d. Ants of the genus Ponera in America, north of Mexico. Ann. Entomol. Soc. Am. 29: 420-430.
- Taylor, R. W. 1967a. A monographic revision of the ant genus Ponera Latreille (Hymenoptera: Formicidae). Pac. Insects Monogr. 13: 1-112 (page 11, Combination in Hypoponera)
- Van Pelt, A. F. 1958. The ecology of the ants of the Welaka Reserve, Florida (Hymenoptera: Formicidae). Part II. Annotated list. American Midland Naturalist. 59:1-57.
- Varela-Hernández, F., Medel-Zosayas, B., Martínez-Luque, E.O., Jones, R.W., De la Mora, A. 2020. Biodiversity in central Mexico: Assessment of ants in a convergent region. Southwestern Entomologist 454: 673-686.
- Wetterer, J.K. 2017. Invasive ants of Bermuda revisited. Journal of Hymenoptera Research 54, 33–41 (doi:10.3897/jhr.54.11444).
- Wetterer, J.K. 2021. Ants (Hymenoptera, Formicidae) of St. Vincent, West Indies. Sociobiology 68, e6725 (doi:10.13102/sociobiology.v68i2.6725).
- Wetterer, J.K., Wetterer, A.L. 2004. Ants (Hymenoptera: Formicidae) of Bermuda. Florida Entomologist 87(2), 212–221 (doi:10.1653/0015-4040(2004)087[0212:ahfob2.0.CO;2]).
- Wheeler, G. C.; Wheeler, J. 1964b. The ant larvae of the subfamily Ponerinae: supplement. Ann. Entomol. Soc. Am. 57: 443-462 (page 453, larva described)
- Wheeler, W. M. 1937c. Mosaics and other anomalies among ants. Cambridge, Mass.: Harvard University Press, 95 pp. (page 59, see also)
- Wilson, E. O.; Taylor, R. W. 1967b. The ants of Polynesia (Hymenoptera: Formicidae). Pac. Insects Monogr. 14: 1-109 (page 28, Senior synonym of perkinsi (and its junior synonym andrei))
References based on Global Ant Biodiversity Informatics
- Achury R., and A.V. Suarez. 2017. Richness and composition of ground-dwelling ants in tropical rainforest and surrounding landscapes in the Colombian Inter-Andean valley. Neotropical Entomology https://doi.org/10.1007/s13744-017-0565-4
- Alayo D. P. 1974. Introduccion al estudio de los Himenopteros de Cuba. Superfamilia Formicoidea. Academia de Ciencias de Cuba. Instituto de Zoologia. Serie Biologica no.53: 58 pp. La Habana.
- Barberena-Arias M. F., and T. M. Aide. 2003. Species Diversity and Trophic Composition of Litter Insects During Plant Secondary Succession. Caribbean Journal of Science 39(2): 161-169.
- Basset Y., L. Cizek, P. Cuenoud, R. K. Didham, F. Guilhaumon, O. Missa, V. Novotny, F. Odegaards, T. Roslin, J. Schmidl et al. 2012. Arthropod diversity in a tropical forest. Science 338(6113): 1481-1484.
- Boer P. 2019. Ants of Curacao, species list. Accessed on January 22 2019 at http://www.nlmieren.nl/websitepages/SPECIES%20LIST%20CURACAO.html
- Boer P. 2019. Ants of Saba, species list. Accessed on January 22 2019 at http://www.nlmieren.nl/websitepages/SPECIES%20LIST%20SABA.html
- Borgmeier T. 1923. Catalogo systematico e synonymico das formigas do Brasil. 1 parte. Subfam. Dorylinae, Cerapachyinae, Ponerinae, Dolichoderinae. Archivos do Museu Nacional (Rio de Janeiro) 24: 33-103.
- Bruch C. 1915. Suplemento al catálogo de los formícidos argentinos. I. (Addenda et corrigenda). Revista del Museo de La Plata 19: 527-537.
- Cuezzo, F. 1998. Formicidae. Chapter 42 in Morrone J.J., and S. Coscaron (dirs) Biodiversidad de artropodos argentinos: una perspectiva biotaxonomica Ediciones Sur, La Plata. Pages 452-462.
- Dash S. T. 2011. A taxonomic revision of the New World Hypoponera Santschi, 1938 (Hymenoptera: Formicidae). PhD Thesis University of Texas at El Paso, 296 pages.
- Emery C. 1906. Studi sulle formiche della fauna neotropica. XXVI. Bullettino della Società Entomologica Italiana 37: 107-194.
- Emery C. 1911. Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1-125.
- Escalante Gutiérrez J. A. 1993. Especies de hormigas conocidas del Perú (Hymenoptera: Formicidae). Revista Peruana de Entomología 34:1-13.
- Fernández F., and T. M. Arias-Penna. 2008. Las hormigas cazadoras en la región Neotropical. Pp. 3-39 in: Jiménez, E.; Fernández, F.; Arias, T.M.; Lozano-Zambrano, F. H. (eds.) 2008. Sistemática, biogeografía y conservación de las hormigas cazadoras de Colombia. Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, xiv + 609 pp.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Fleck M. D., E. Bisognin Cantarelli, and F. Granzotto. 2015. Register of new species of ants (Hymenoptera: Formicidae) in Rio Grande do Sul state. Ciencia Florestal, Santa Maria 25(2): 491-499.
- Fontanla Rizo J.L. 1997. Lista preliminar de las hormigas de Cuba. Cocuyo 6: 18-21.
- Fontenla J. L., and J. Alfonso-Simonetti. 2018. Classification of Cuban ants (Hymenoptera: Formicidae) into functional groups. Poeyana Revista Cubana de Zoologia 506: 21-30.
- Fontenla Rizo J. L. 1993. Mirmecofauna de Isla de la Juventud y de algunos cayos del archipielago cubano. Poeyana. Instituto de Ecologia y Sistematica, Academia de Ciencias de Cuba 444:1-7.
- Fontenla Rizo J. L. 1997. Lista preliminar de las hormigas de Cuba (Hymenoptera: Formicidae). Cocuyo 6: 18-21.
- Forel A. 1908. Ameisen aus Sao Paulo (Brasilien), Paraguay etc. gesammelt von Prof. Herm. v. Ihering, Dr. Lutz, Dr. Fiebrig, etc. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 58: 340-418.
- Forster J.A. 2005. The Ants (hymenoptera: Formicidae) of Alabama. Master of Science, Auburn University. 242 pages.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Gallardo A. 1918. Las hormigas de la República Argentina. Subfamilia Ponerinas. Anales del Museo Nacional de Historia Natural de Buenos Aires 30: 1-112.
- Garcia M. A. The vulnerability of leaflitter ants to forest disturbances in the islands of Puerto Rico, Greater Antilles. Novitates Caribaea 13: 74-91.
- Kempf W. W. 1960. Insecta Amapaensia. - Hymenoptera: Formicidae (segunda contribuição). Studia Entomologica (n.s.)3: 385-400.
- Kempf W. W. 1962. Miscellaneous studies on neotropical ants. II. (Hymenoptera, Formicidae). Studia Entomologica 5: 1-38.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Kusnezov N. 1963. Zoogeografia de las hormigas en sudamerica. Acta Zoologica Lilloana 19: 25-186
- Kusnezov N. 1978. Hormigas argentinas: clave para su identificación. Miscelánea. Instituto Miguel Lillo 61:1-147 + 28 pl.
- Leponce, M., L. Theunis, J.H.C. Delabie and Y. Roisin. 2004. Scale dependence of diversity measures in a leaf-litter ant assemblage. Ecography. 27:253-267.
- Lutinski J. A., B. C. Lopes, and A. B. B.de Morais. 2013. Diversidade de formigas urbanas (Hymenoptera: Formicidae) de dez cidades do sul do Brasil. Biota Neotrop. 13(3): 332-342.
- Maes, J.-M. and W.P. MacKay. 1993. Catalogo de las hormigas (Hymenoptera: Formicidae) de Nicaragua. Revista Nicaraguense de Entomologia 23.
- Mann W. M. 1920. Additions to the ant fauna of the West Indies and Central America. Bulletin of the American Museum of Natural History 42: 403-439.
- Menozzi C, Russo G. 1930. Contributo alla conoscenza della mirmecofauna della Repubblica Dominicana (Antille). Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d'Agricoltura. Portici. 24: 148-173.
- Morrison L. W. 1998. A review of Bahamian ant (Hymenoptera: Formicidae) biogeography. Journal of Biogeography 25: 561-571.
- Osorio-Perez K., M. F. Barberena-Arias, and T. M. Aide. 2007. Changes in Ant Species Richness and Composition During Plant Secondary Succession in Puerto Rico. Caribbean Journal of Science 43(2): 244-253.
- Pereira M. C., J. H. C. Delabie, Y. R. Suarez, and W. F. Antonialli Junior. 2013. Spatial connectivity of aquatic macrophytes and flood cycle influence species richness of an ant community of a Brazilian floodplain. Sociobiology 60(1): 41-49.
- Perez-Gelabert D. E. 2008. Arthropods of Hispaniola (Dominican Republic and Haiti): A checklist and bibliography. Zootaxa 1831:1-530.
- Portuondo E. F., and J. L. Reyes. 2002. Mirmecofauna de los macizos montañosos de Sierra Maestra y Nipe-Sagua-Baracoa. Cocuyo 12: 10-13
- Portuondo Ferrer E., and J. L. Fernández Triana. 2005. Species of hymenopterans (bees, wasps, and ants) recorded in Alejandro de Humboldt National Park, from literature records, revision of the collection at BIOECO, and collections before and during the rapid inventory, 12-22 February 2004. In Fong G., A., D. Maceira F., W. S. Alverson, y/and T. Wachter, eds. 2005. Cuba: Parque Nacional Alejandro de Humboldt. Rapid Biological Inventories Report 14. The Field Museum, Chicago.
- Portuondo Ferrer, E. and J. Fernandez Triana. Biodiversidad del orden Hymenoptera en Los Macizos Montanosos de Cuba Oriental. Boletin S.E.A. 35:121-136.
- Reyes, J. L.. "Inventario de la colección de hormigas (Hymenoptera: Formicidae) del Centro Oriental de Ecosistemas y Biodiversidad, Santiago de Cuba, Cuba." Boletín de la Sociedad Aragonesa 36 (2005): 279-283.
- Rosa da Silva R. 1999. Formigas (Hymenoptera: Formicidae) do oeste de Santa Catarina: historico das coletas e lista atualizada das especies do Estado de Santa Catarina. Biotemas 12(2): 75-100.
- Santoandre S., J. Filloy, G. A. Zurita, and M. I. Bellocq. 2019. Ant taxonomic and functional diversity show differential response to plantation age in two contrasting biomes. Forest Ecology and Management 437: 304-313.
- Santos P. P., A. Vasconcelos, B. Jahyny, and J. H. C. Delabie. 2010. Ant fauna (Hymenoptera, Formicidae) associated to arboreal nests of Nasutitermes spp. (Isoptera, Termitidae) in a cacao plantation in southeastern Bahia, Brazil. Revista Brasileira de Entomologia 54(3): 450-454.
- Santschi F. 1925. Nouvelles fourmis brésiliennes. Annales de la Société Entomologique de Belgique. 64: 5-20.
- Smith M. R. 1936. Ants of the genus Ponera in America, north of Mexico. Annals of the Entomological Society of America 29: 420-430.
- Smith M. R. 1937. The ants of Puerto Rico. Journal of Agriculture of the University of Puerto Rico 20: 819-875.
- Taylor R. W. 1987. A checklist of the ants of Australia, New Caledonia and New Zealand (Hymenoptera: Formicidae). CSIRO (Commonwealth Scientific and Industrial Research Organization) Division of Entomology Report 41: 1-92.
- Torres, Juan A. and Roy R. Snelling. 1997. Biogeography of Puerto Rican ants: a non-equilibrium case?. Biodiversity and Conservation 6:1103-1121.
- Vasconcelos, H.L., J.M.S. Vilhena, W.E. Magnusson and A.L.K.M. Albernaz. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. Journal of Biogeography 33:1348-1356
- Vittar, F. 2008. Hormigas (Hymenoptera: Formicidae) de la Mesopotamia Argentina. INSUGEO Miscelania 17(2):447-466
- Vittar, F., and F. Cuezzo. "Hormigas (Hymenoptera: Formicidae) de la provincia de Santa Fe, Argentina." Revista de la Sociedad Entomológica Argentina (versión On-line ISSN 1851-7471) 67, no. 1-2 (2008).
- Weber N. A. 1948. Studies on the fauna of Curaçao, Aruba, Bonaire and the Venezuelan islands: No. 14. Ants from the Leeward Group and some other Caribbean localities. Natuurwetenschappelijke Studiekring voor Suriname en de Nederlandse Antillen 5: 78-86.
- Wheeler W. M. 1905. The ants of the Bahamas, with a list of the known West Indian species. Bulletin of the American Museum of Natural History 21: 79-135.
- Wheeler W. M. 1908. The ants of Porto Rico and the Virgin Islands. Bulletin of the American Museum of Natural History 24: 117-158.
- Wheeler W. M. 1913. The ants of Cuba. Bulletin of the Museum of Comparative Zoology 54: 477-505.
- Wheeler W. M. 1916. Ants collected in British Guiana by the expedition of the American Museum of Natural History during 1911. Bulletin of the American Museum of Natural History 35: 1-14.
- Wheeler W. M. 1917. Jamaican ants collected by Prof. C. T. Brues. Bulletin of the Museum of Comparative Zoology 61: 457-471.
- Wheeler W. M. 1922. The ants of Trinidad. American Museum Novitates 45: 1-16.
- Wheeler W. M. 1925. Neotropical ants in the collections of the Royal Museum of Stockholm. Arkiv för Zoologi 17A(8): 1-55.
- Wheeler W. M., and W. M. Mann. 1914. The ants of Haiti. Bulletin of the American Museum of Natural History 33: 1-61.
- Wheeler, William Morton. 1911. Additions to the Ant-Fauna of Jamaica. Bulletin American Museum of Natural History. 30:21-29.
- Wheeler, William Morton. 1916. Ants Collected in Trinidad by Professor Roland Thaxter, Mr. F. W. Urich, and Others. Bulletin of the Museum of Comparitive Zoology at Harvard University. 40(8):322-330
- Wheeler, William Morton. 1936. Ants From Hispaniola and Mona Island. Bulletin: Museum of Comparative Zoology at Harvard College. 80(2):192-211.
- Wild, A. L.. "A catalogue of the ants of Paraguay (Hymenoptera: Formicidae)." Zootaxa 1622 (2007): 1-55.
- Zolessi L. C. de, Y. P. Abenante, and M. E. de Philippi. 1988. Lista sistematica de las especies de Formicidos del Uruguay. Comun. Zool. Mus. Hist. Nat. Montev. 11: 1-9.
- Zolessi L. C. de; Y. P. de Abenante, and M. E. Philippi. 1989. Catálogo sistemático de las especies de Formícidos del Uruguay (Hymenoptera: Formicidae). Montevideo: ORCYT Unesco, 40 + ix pp.
- de Zolessi, L.C., Y.P. de Abenante and M.E. Philippi. 1987. Lista sistemática de las especies de formícidos del Uruguay. Comunicaciones Zoologicas del Museo de Historia Natural de Montevideo 11(165):1-9

