Crematogaster crinosa
| Crematogaster crinosa | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Crematogastrini |
| Genus: | Crematogaster |
| Species: | C. crinosa |
| Binomial name | |
| Crematogaster crinosa Mayr, 1862 | |
| Synonyms | |
| |
A widespread, common species that can form large colonies. Crematogaster crinosa is found across a range of habitats but the largest colonies are found in dryer areas such as seasonally dry forest. Their polydomous nests can be found in just about all the available cavities, i.e., any protected space, in the trees they occupy.
Identification
Longino (2003) - Members of the crinosa-complex are among the most frequently encountered Neotropical ants, particularly in open or seasonally dry habitats. They are geographically variable and taxonomically difficult, and species boundaries are poorly defined. Crematogaster crinosa, Crematogaster rochai, and Crematogaster torosa are three very similar species that occur together in Costa Rica. They are difficult to distinguish and workers may not always be clearly identified. All three have the face with sparse erect setae over short appressed pubescence, the mesosomal dorsum and fourth abdominal tergite with short, stiff erect setae (or erect setae absent), the dorsal face of the petiole short with convex sides, and the propodeal spines short and upturned. Crematogaster crinosa can be differentiated from rochai throughout the range, because crinosa has a dense, even covering of erect setae on the fourth abdominal tergite, while rochai completely lacks these setae or has only a small cluster on each anterolateral humerus. Distinguishing crinosa from torosa is more difficult. In Costa Rica, torosa also has abundant erect setae on the fourth abdominal tergite, but these are usually clustered laterally and anterolaterally, leaving a median strip free of setae. Also, crinosa always has a long, sharp anteroventral petiolar process, while torosa more often has a short, blunt or squared-off process. Crematogaster crinosa can also be confused with Crematogaster erecta and Crematogaster moelleri, but these have flexuous erect setae on the pronotal humeri.
Keys including this Species
Distribution
Longino (2003) - Throughout the Neotropics, from southern Texas to Argentina and on numerous Caribbean islands.
Latitudinal Distribution Pattern
Latitudinal Range: 38.74027778° to -36.2°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Nearctic Region: United States.
Neotropical Region: Argentina, Barbados, Belize, Bolivia, Brazil (type locality), Chile, Colombia, Costa Rica, Ecuador, French Guiana, Greater Antilles, Grenada, Guatemala, Guyana, Honduras, Lesser Antilles, Martinique, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Trinidad and Tobago, Uruguay, Venezuela.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
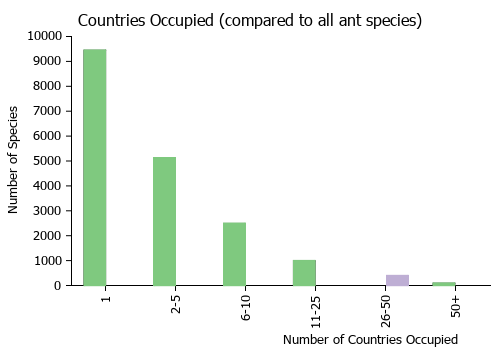
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Biology
Longino (2003) - Crematogaster crinosa is an extremely widespread and generalized species that prefers highly insolated habitats. It is common in seasonally dry areas, less common in wet forests. In wet forest habitats it is typically found in the high canopy or in disturbed areas. It may form monodominant populations in mangrove forests.
Colonies are large and polydomous and it is usually difficult to locate colony boundaries. Nests are found in almost any kind of cavity, and columns of workers move from nest to nest. Nests can be in live or dead branches, in small rotten knots, under bark flaps, in cavities in fence posts, opportunistically in ant plants, and thinly dispersed in multiple small bark cavities. Workers, brood, and alate sexuals are dispersed across nests. Small amounts of carton construction are used to form baffles inside of nest cavities and to restrict nest entrances, but large external carton nests are never constructed.
Although new alate queens are relatively common in nests, I have rarely encountered physogastric colony queens. In my collecting experience, I have never found a colony that was obviously polygynous, with many dealate queens dispersed in many nests. However, I am treating Forel's minutior as a synonym of crinosa, and minutior from St. Vincent Island in the West Indies forms large polygynous, polydomous colonies in coastal areas (Forel 1893).
In Colombia I observed the beginning of a nuptial flight just after dusk. I found a dense aggregation of males and workers under a bark flap, and the males were just beginning to fly.
Workers are omnivorous. They are attracted to protein and carbohydrate baits, they scavenge dead or injured insects, they visit extrafloral nectaries, and they tend Homoptera. When nests are disturbed they can be aggressive and will bite. Workers are continuously polymorphic, with a broad range of worker sizes.
Ecological equivalents are torosa and rochai. I can detect very few behavioral or ecological differences among these species. Crematogaster crinosa is the only member of the group that regularly dominates mangrove habitats. Mangrove forests in Costa Rica are sometimes dominated by Azteca, sometimes by C. crinosa. I found a similar situation in the Santa Marta area of Colombia. I have only one record of rochai from mangroves (a voucher collection from Adams' studies of mangrove communities, Adams 1994), and I have no record of torosa from mangroves. Other than in mangroves, crinosa is less abundant relative to torosa or rochai. For example, a collecting trip to a wildlife refuge in southern Texas yielded 13 separate collections of torosa but only one of crinosa. In northwestern Costa Rica, torosa and rochai are far more abundant than crinosa. Based on museum collections, crinosa seems to be the most common member of the crinosa group on various Caribbean and Pacific islands.
Gillette et al. (2015) in a Chaipas, Mexico field study of twig-nesting ants in coffee plants found C. crinosa nesting on plants between 1150-1450 m in elevation.
Philpot et al. (2018) reported this species was one of the most common ants in an experimental study examining colonization of twigs in shade coffee forests in Chiapas, Mexico (9.4% of the 202 nests found in 796 recovered twigs).
Association with Other Organisms
 Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
Explore: Show all Associate data or Search these data. See also a list of all data tables or learn how data is managed.
- This species is a host for the eulophid wasp Melittobia australica (a parasite) (Universal Chalcidoidea Database) (primary host).
- This species is a host for the eulophid wasp Melittobia australica (a parasitoid) (Quevillon, 2018) (multiple encounter modes; direct transmission; transmission outside nest).
- This species is a prey for the syrphid fly Stipomorpha wheeleri (a predator) (Quevillon, 2018).
Castes
Worker
Images from AntWeb
   
| |
| Lectotype of Crematogaster brevispinosa chathamensis. Worker. Specimen code castype03689. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by CAS, San Francisco, CA, USA. |
   
| |
| Worker. Specimen code jtlc000001769. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by JTLC. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- crinosa. Crematogaster crinosa Mayr, 1862: 767 (w.) BRAZIL (Rio de Janeiro).
- Type-material: syntype workers (number not stated).
- Type-locality: Brazil: Rio de Janeiro (Novara Expd.).
- Type-depository: NMHW.
- Mayr, 1887: 627 (q.m.).
- Combination in C. (Orthocrema): Emery, 1922e: 134.
- Status as species: Mayr, 1863: 404; Roger, 1863b: 37; Mayr, 1865: 104 (redescription); Mayr, 1870b: 992 (in key); Mayr, 1884: 37; Mayr, 1887: 626; Emery, 1888c: 356; Dalla Torre, 1893: 80; von Jhering, 1894: 394; Forel, 1895b: 131; Forel, 1908c: 366; Borgmeier, 1927c: 93; Kempf, 1972a: 86; Bolton, 1995b: 151; Longino, 2003a: 49 (redescription), 126; Wild, 2007b: 32; Branstetter & Sáenz, 2012: 258; Bezděčková, et al. 2015: 116; Wetterer, et al. 2016: 10; Morgan & Mackay, 2017: 124 (redescription); Pedraza & Fernández, 2019: 895.
- Senior synonym of brevispinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Senior synonym of chathamensis: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Senior synonym of minutior: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Senior synonym of recurvispina: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Senior synonym of sampaioi: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Senior synonym of schuppi: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Senior synonym of striatinota: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Senior synonym of townsendi: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- Material of the unavailable name semisericea referred here by Longino, 2003a: 49.
- Distribution: Argentina, Barbados, Belize, Bolivia, Brazil, Colombia, Costa Rica, Ecuador (+ Galapagos Is), French Guiana, Grenada, Guatemala, Guyana, Honduras, Jamaica, Mexico, Nicaragua, Panama, Paraguay, Peru, San Salvador, St Vincent & the Grenadines, Suriname, Trinidad, U.S.A., Venezuela.
- brevispinosa. Crematogaster brevispinosa Mayr, 1870a: 403 (w.) COLOMBIA (“New Granada”).
- Type-material: holotype worker.
- Type-locality: Colombia (“New Granada”): Santa Fé de Bogotá (Lindig).
- Type-depository: NHMW.
- Wheeler, G.C. & Wheeler, J. 1952b: 260 (l.).
- Combination in C. (Orthocrema): Santschi, 1918d: 182.
- Status as species: Mayr, 1870b: 992 (in key); Mayr, 1884: 37; Mayr, 1887: 626; Emery, 1890b: 53; Dalla Torre, 1893: 80; Pergande, 1894: 165; Emery, 1894k: 57; Emery, 1896h: 625; Forel, 1899c: 84; Emery, 1906c: 140; Wheeler, W.M. 1907a: 272; Forel, 1907e: 6; Forel, 1908b: 47; Forel, 1909a: 258; Wheeler, W.M. 1911a: 28; Wheeler, W.M. 1911b: 169; Forel, 1912f: 211; Santschi, 1913h: 36; Wheeler, W.M. 1913d: 241; Forel, 1914e: 11; Wheeler, W.M. 1916c: 12; Mann, 1916: 443; Luederwaldt, 1918: 41; Wheeler, W.M. 1922c: 8; Mann, 1922: 29; Emery, 1922e: 134; Wheeler, W.M. 1923c: 4; Wheeler, W.M. 1925a: 24; Essig, 1926: 860; Borgmeier, 1927c: 92; Menozzi, 1927c: 267; Santschi, 1929d: 293; Menozzi, 1931b: 267; Gallardo, 1934: 11 (redescription); Menozzi, 1935b: 195; Wheeler, W.M. 1942: 194; Kempf, 1972a: 84; Zolessi, et al. 1988: 4; Brandão, 1991: 338; Bolton, 1995b: 149.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- chathamensis. Crematogaster (Orthocrema) brevispinosa subsp. chathamensis Wheeler, W.M. 1933a: 58 (w.) ECUADOR (Galapagos Is).
- Type-material: lectotype worker (by designation of Longino, 2003a: 50), 9 paralectotype workers.
- Type-locality: lectotype Ecuador: Galapagos Is, Chatham I., 17.iv.1932 (M. Willows); paralectotypes with same data.
- Type-depositories: CASC (lectotype); CASC, MCZC (paralectotypes).
- Subspecies of brevispinosa: Wheeler, W.M. 1935g: 21; Linsley & Usinger, 1966: 175; Kempf, 1972a: 85; Bolton, 1995b: 150.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- minutior. Crematogaster brevispinosa var. minutior Forel, 1893g: 399 (w.q.) ST VINCENT & THE GRENADINES (St Vincent I.).
- Type-material: syntype workers (number not stated).
- Type-localities: St Vincent & the Grenadines: St Vincent I., Richmond Estate, 14.x., no. 7a (H.H. Smith), St Vincent I., Villa Estate, 20.xi., no 7d (H.H. Smith).
- Combination in C. (Orthocrema): Emery, 1922e: 134.
- Subspecies of brevispinosa: Forel, 1897b: 300; Forel, 1901c: 129; Wheeler, W.M. 1905b: 126; Wheeler, W.M. 1911a: 28; Wheeler, W.M. 1911b: 169; Forel, 1912f: 211; Wheeler, W.M. 1922c: 8; Emery, 1922e: 134; Santschi, 1925d: 230; Borgmeier, 1927c: 92; Wheeler, W.M. 1933a: 62; Wheeler, W.M. 1934f: 138; Wheeler, W.M. 1935g: 21; Wheeler, W.M. 1942: 194; Kempf, 1972a: 85; Bolton, 1995b: 157.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- recurvispina. Crematogaster brevispinosa r. recurvispina Forel, 1912f: 212 (w.) BRAZIL (Rio de Janeiro).
- Type-material: syntype workers (number not stated).
- Type-locality: Brazil: Rio de Janeiro (Sampaio, Naegeli).
- Type-depository: MHNG.
- [Misspelled as recurvispinosa by Emery, 1922e: 134.]
- Combination in C. (Orthocrema): Emery, 1922e: 134; Santschi, 1925d: 230.
- Subspecies of brevispinosa: Emery, 1922e: 134; Santschi, 1925d: 230; Borgmeier, 1927c: 93; Kempf, 1972a: 85; Bolton, 1995b: 161.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- sampaioi. Crematogaster brevispinosa r. sampaioi Forel, 1912f: 213 (w.q.m.) BRAZIL (Rio de Janeiro).
- Type-material: syntype workers, syntype queens, syntype males (numbers not stated).
- Type-locality: Brazil: Rio de Janeiro (Sampaio).
- Type-depository: MHNG.
- Combination in C. (Orthocrema): Emery, 1922e: 134.
- Subspecies of brevispinosa: Emery, 1922e: 134; Borgmeier, 1927c: 93; Kempf, 1972a: 86; Bolton, 1995b: 162.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- schuppi. Crematogaster brevispinosa var. schuppi Forel, 1901d: 299 (w.q.) BRAZIL (Rio Grande do Sul).
- Type-material: syntype workers (number not stated), 1 syntype queen.
- Type-locality: Brazil: Rio Grande do Sul, Porto Alegre (Schupp).
- Type-depository: MHNG.
- Combination in C. (Orthocrema): Emery, 1922e: 134.
- As unavailable (infrasubspecific) name: Forel, 1908e: 69; Borgmeier, 1927c: 92; Kempf, 1972a: 85.
- Subspecies of brevispinosa: Forel, 1911e: 273; Forel, 1912f: 212; Luederwaldt, 1918: 41; Emery, 1922e: 134; Bolton, 1995b: 162.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- striatinota. Crematogaster brevispinosa var. striatinota Forel, 1912f: 211 (w.) COLOMBIA.
- Type-material: syntype workers (number not stated).
- Type-localities: Colombia: Magdalena, Santa Marta (A. Forel), Colombia: Barranquilla (A. Forel).
- Type-depository: MHNG.
- As unavailable (infrasubspecific) name: Kempf, 1972a: 85.
- Subspecies of brevispinosa: Emery, 1922e: 134; Bolton, 1995b: 163.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
- townsendi. Crematogaster (Orthocrema) brevispinosa subsp. townsendi Wheeler, W.M. 1925a: 25 (w.) PERU.
- Type-material: syntype workers (number not stated, “a number”).
- Type-locality: Peru: Piura (C.H.T. Townsend).
- Type-depositories: MCZC, NHRS.
- Subspecies of brevispinosa: Kempf, 1972a: 86; Bolton, 1995b: 164.
- Junior synonym of crinosa: Longino, 2003a: 49; Morgan & Mackay, 2017: 124.
Unless otherwise noted the text for the remainder of this section is reported from the publication that includes the original description.
Longino (2003) - Synonyms: The workers of Crematogaster recurvispina are small, with few setae on mesosoma and fourth abdominal tergite, and a strong anteroventral petiolar tooth. These may just be small workers of crinosa. A brief examination of the schuppi types revealed a queen and a minim worker. The queen had a strong anteroventral petiolar tooth, was abundantly setose, and had a quadrate head. It is probably crinosa. The other synonymies are all based on examination of medium-size to large workers that match the general features of crinosa as defined here.
Description
Worker
Wheeler (1933) for Crematogaster brevispinosa chathamensis - Length 2.5-3.3 mm.
Head slightly broader than long in the largest specimens, with slightly concave posterior border. Eyes elongate, flattened, near the middle of the sides. Clypeus convex, with nearly straight, transverse anterior border. Antennal scapes not reaching to the posterior border of the head; funiculi with distinctly 2•jointed club; basal funicular joints, except the first, broader than long. Thorax short; pro- and mesonotum convex and hemispherical; mesoepinotal impression short and deep; base of epinotum short, anteriorly very convex and rising rather abruptly from the impression; declivity much longer, sloping; spines suberect, much shorter than their distance apart at the base, their tips slender and acute, sometimes slightly recurved. Petiole somewhat longer than broad, subelliptical, as broad behind as in front, with rounded sides and distinctly dentate posterior corners, anteroventrally with a strong spine, directed forward and downward. Postpetiole short and convex, narrower than the petiole, without median dorsal groove. Legs rather short and stout.
Mandibles, clypeus, front, gula and sides of head finely, longitudinally striate; posterior portion of head smooth and shining, with sparse piligerous punctures. Thorax subopaque, only the epinotal declivity shining; pronotum transversely, mesonotum and base of epinotum longitudinally striate; meso- and metapleurae evenly and densely punctate. Petiole and postpetiole shining, the former smooth above and coarsely reticulate below, the latter delicately longitudinally rugulose. Gaster subopaque, finely punctate.shagreened.
Hairs pale, sparse, blunt and erect on the thorax, pedicel and gaster; head, scapes and gaster with long, sparse, appressed pubescence; legs with similar but finer pubescence.
Large workers red, with the thorax and posterior portion of the gaster black; trochanters and tarsi yellow; smaller workers darker, blackish, with mandibles, antenne, tibiae and tarsi reddish; trochanters yellow.
Longino (2003) - HL 0.801, 0.616, 1.052; HW 0.869, 0.701, 1.156; HC 0.837, 0.664, 1.123; SL 0.537, 0.454, 0.697; EL 0.175, 0.147, 0.252; A11L 0.248; A11W 0.138; A10L 0.097; A10W 0.113; A09L 0.055; A09W 0.078; A08L 0.034; A08W 0.066; WL 0.844, 0.688, 1.146; SPL 0.134, 0.095, 0.168; PTH 0.174, 0.142, 0.203; PTL 0.239, 0.206, 0.343; PTW 0.253, 0.224, 0.323; PPL 0.198, 0.182, 0.254; PPW 0.246, 0.205, 0.328; CI 108, 114, 110; OI 22, 24, 24; SI 67, 74, 66; PTHI 73, 69, 59; PTWI 106, 109, 94; PPI 124, 113, 129; SPI 16, 14, 15; ACI 0.64.
Color red brown to black; workers usually with pronounced size polymorphism. In face view head subquadrate, wider than long in larger workers, with emarginate posterior margin; mandibles coarsely striate, striae faint to pronounced; clypeus smooth and shiny or faintly granular or finely longitudinally striate; scapes short, in face view not attaining posterior margin of head when laid back; terminal three segments of antenna gradually lengthening and broadening, becoming increasingly densely pubescent, terminal two segments very much larger, so that antennal club appears two-segmented; scapes with short appressed pubescence, sometimes subdecumbent, never erect, with no differentiated long erect setae (occasionally a long seta on very large workers); face with sparse appressed to subdecument pubescence and sparse short erect setae; face smooth and shining or with variably developed fine longitudinal striation, most common on anterior face and space between eye and antennal insertion, occasionally extending posteriorly and medially, but always with at least median strip sublucid.
Promesonotal profile forming a single, somewhat flat-topped convexity; in large workers promesonotal suture visible, a dorsolateral arch that extends far forward, showing that dorsal pronotum is short and much of promesonotal dorsum composed of mesonotum (approaching queen condition); in small workers promesonotal suture effaced, visible only as oblique anterolateral impressions; propodeum with short but distinctly differentiated dorsal face, such that propodeal suture distinctly visible in lateral view as v-shaped impression; propodeum with long sloping posterior face; propodeal spines short, upturned; promesonotal dorsum and dorsal face of propodeum faintly punctate with varying development of longitudinal or transversely whorled rugulae or striations, lateral carinulae bridge propodeal suture, rarely forming a small triangular denticle; posterior face of propodeum smooth and shining or faintly microareolate; lateral pronotum with faint microsculpture; katepisternum and lateral propodeum faintly punctate to microareolate; promesonotum and bases of propodeal spines with highly variable number but usually abundant short stiff flattened setae; femora and tibiae with appressed to subdecumbent pubescence, no erect setae.
Petiole in lateral view subtriangular, often with slightly concave ventral margin, with strongly developed, anteriorly projecting, acute anteroventral tooth; side faintly granular or microareolate; dorsal face of petiole smooth and shining to faintly microareolate, about as wide as long, subquadrate or more often with convex sides, widest about one third distance from anterior margin, with one or more stiff setae on posterolateral tubercles; postpetiole with no ventral tooth, in dorsal view globular to subquadrate, usually slightly broader than long, rarely with faintly impressed posteromedian sulcus, with four or more stiff setae; fourth abdominal tergite smooth and shining or faintly microareolate, with abundant vestiture of short, stiff, flattened, erect setae, evenly distributed over surface of tergite (not clustered or concentrated anterolaterally).
Queen
Longino (2003) - A normal queen (dorsal face of propodeum drops steeply from postscutellum and much of propodeum appears ventral to scutellum and postscutellum) with general shape, sculpture, and pilosity characters of the worker; size characters as in Figures.
Type Material
Crematogaster brevispinosa chathamensis Described from ten workers taken on Chatham Island (IV.17.'32). Lectotype, C. A. S. Ent. No. 3689.
Longino (2003) - Syntype workers: Brazil, Rio de Janeiro (Novara) Naturhistorisches Museum Wien, Vienna (examined).
References
- Aguilar-Méndez, M.J., Rosas-Mejía, M., Vásquez-Bolaños, M., González-Hernández, G.A., Janda, M. 2021. New distributional records for ants and the evaluation of ant species richness and endemism patterns in Mexico. Biodiversity Data Journal 9, e60630 (doi:10.3897/bdj.9.e60630).
- Alatorre-Bracamontes, C.E., Vásquez-Bolaños, M. 2010. Lista comentada de las hormigas (Hymenoptera: Formicidae) del norte de México. Dugesiana 17(1): 9-36.
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Emery, C. 1922c. Hymenoptera. Fam. Formicidae. Subfam. Myrmicinae. [part]. Genera Insectorum 174B: 95-206 (page 134, Combination in C. (Orthocrema))
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Gillette, P. N., K. K. Ennis, G. D. Martinez, and S. M. Philpott. 2015. Changes in Species Richness, Abundance, and Composition of Arboreal Twig-nesting Ants Along an Elevational Gradient in Coffee Landscapes. Biotropica. 47:712-722. doi:10.1111/btp.12263
- Hernández‐Flores, J., Flores‐Palacios, A., Vásquez‐Bolaños, M., Toledo‐Hernández, V.H., Sotelo‐Caro, O., Ramos‐Robles, M. 2020. Effect of forest disturbance on ant (Hymenoptera: Formicidae) diversity in a Mexican tropical dry forest canopy. Insect Conservation and Diversity (doi:10.1111/icad.12466).
- Longino, J.T. 2003a. The Crematogaster of Costa Rica. Zootaxa 151: 1-150. (page 49, Senior synonym of brevispinosa, minutior, schuppi, stratinota, recurvispina, sampaioi, semisericea and chathamensis;page 50, worker, queen described)
- López-Dávila, A.J., Escobar-Ramírez, S., Armbrecht, I. 2021. Nesting of arboreal ants (Hymenoptera: Formicidae) in artificial substrates in coffee plantations in the Colombian Andes. Uniciencia 35, 1–18 (doi:10.15359/ru.35-2.13).
- MacGown, J.A., Booher, D., Richter, H., Wetterer, J.K., Hill, J.G. 2021. An updated list of ants of Alabama (Hymenoptera: Formicidae) with new state records. Transactions of the American Entomological Society 147: 961-981 (doi:10.3157/061.147.0409).
- Mayr, G. 1862. Myrmecologische Studien. Verh. K-K. Zool.-Bot. Ges. Wien 12: 649-776 (page 767, worker described)
- Mayr, G. 1887. Südamerikanische Formiciden. Verh. K-K. Zool.-Bot. Ges. Wien 37: 511-632 (page 627, queen, male described)
- Morgan, C.E., Mackay, W.P. 2017. The North American Acrobat Ants of the hyperdiverse genus Crematogaster (Hymneoptera: Formicidae). Lambert Academic Publishing (PDF version, 532 pp.)
- Narváez-Vásquez, A., Gaviria, J., Vergara-Navarro, E.V., Rivera-Pedroza, L., Löhr, B. 2021. Ant (Hymenoptera: Formicidae) species diversity in secondary forest and three agricultural land uses of the Colombian Pacific Coast. Revista Chilena de Entomologia 47, 441–458 (doi:10.35249/rche.47.3.21.01).
- Philpott, S. M., Z. Serber, and A. De la Mora. 2018. Influences of Species Interactions With Aggressive Ants and Habitat Filtering on Nest Colonization and Community Composition of Arboreal Twig-Nesting Ants. Environmental Entomology. 47:309-317. doi:10.1093/ee/nvy015
- Prebus, M.M. 2021. Taxonomic revision of the Temnothorax salvini clade (Hymenoptera: Formicidae), with a key to the clades of New World Temnothorax. PeerJ 9, e11514 (doi:10.7717/peerj.11514).
- Varela-Hernández, F., Medel-Zosayas, B., Martínez-Luque, E.O., Jones, R.W., De la Mora, A. 2020. Biodiversity in central Mexico: Assessment of ants in a convergent region. Southwestern Entomologist 454: 673-686.
- Ward, P.S., Blaimer, B.B. 2022. Taxonomy in the phylogenomic era: species boundaries and phylogenetic relationships among North American ants of the Crematogaster scutellaris group (Formicidae: Hymenoptera), Zoological Journal of the Linnean Society 194(3): 893–937 (doi:10.1093/zoolinnean/zlab047).
- Wetterer, J.K. 2021. Ants (Hymenoptera, Formicidae) of St. Vincent, West Indies. Sociobiology 68, e6725 (doi:10.13102/sociobiology.v68i2.6725).
- Wheeler, W. M. 1933a. The Templeton Crocker Expedition of the California Academy of Sciences, 1932. No. 6. Formicidae of the Templeton Crocker Expedition. Proc. Calif. Acad. Sci. (4) 21: 57-64.
References based on Global Ant Biodiversity Informatics
- Adams B. J., S. A. Schnitzer, and S. P. Yanoviak. 2016. Trees as islands: canopy ant species richness increases with the size of liana-free trees in a Neotropical forest. Ecography doi: 10.1111/ecog.02608
- Adams B. J., S. A. Schnitzer, and S. P. Yanoviak. 2019. Connectivity explains local ant community structure in a Neotropical forest canopy: a large-scale experimental approach. Ecology 100(6): e02673.
- Alatorre-Bracamontes, C.E. and M Vasquez-Bolanos. 2010. Lista comentada de las hormigas (Hymenoptera: Formicidae) del norte de México. Dugesiana 17(1):9-36
- Basset Y., L. Cizek, P. Cuenoud, R. K. Didham, F. Guilhaumon, O. Missa, V. Novotny, F. Odegaards, T. Roslin, J. Schmidl et al. 2012. Arthropod diversity in a tropical forest. Science 338(6113): 1481-1484.
- Brandao, C.R.F. 1991. Adendos ao catalogo abreviado das formigas da regiao neotropical (Hymenoptera: Formicidae). Rev. Bras. Entomol. 35: 319-412.
- Castano-Meneses, G. and J.G. Palacio-Vargas. 2003. Effects of fire and agricultural practices on neotropical ant communities. Biodiversity and Conservation 12:1913-1919.
- Castano-Meneses, G., M. Vasquez-Bolanos, J. L. Navarrete-Heredia, G. A. Quiroz-Rocha, and I. Alcala-Martinez. 2015. Avances de Formicidae de Mexico. Universidad Nacional Autonoma de Mexico.
- Cuautle, M. and V. Rico-Gray. 2003. The Effect of Wasps and Ants on the Reproductive Success of the Extrafloral Nectaried Plant Turnera ulmifolia (Turneraceae). Functional Ecology 17(3):417-423
- Dattilo W. et al. 2019. MEXICO ANTS: incidence and abundance along the Nearctic-Neotropical interface. Ecology https://doi.org/10.1002/ecy.2944
- De la Mora, A., C. J. Murnen, and S. M. Philpott. 2013. Local and landscape drivers of ant-communities in Neotropical coffee landscapes. Biodiversity and Conservation 22: 871-888.
- Del Toro, I., M. Vázquez, W.P. Mackay, P. Rojas and R. Zapata-Mata. Hormigas (Hymenoptera: Formicidae) de Tabasco: explorando la diversidad de la mirmecofauna en las selvas tropicales de baja altitud. Dugesiana 16(1):1-14.
- Díaz-Castelazo C., V. Rico-Gray, P. S. Oliveira, and M. Cuautle. 2004. Extrafloral nectary-mediated ant-plant interactions in the coastal vegetation of Veracruz, Mexico: Richness, occurrence, seasonality and ant foraging patterns. Ecoscience 11: 472-481.
- Emery C. 1890. Studii sulle formiche della fauna neotropica. Bull. Soc. Entomol. Ital. 22: 38-8
- Emery C. 1891. Zur Biologie der Ameisen. Biologisches Centralblatt 11: 165-180.
- Emery C. 1894. Estudios sobre las hormigas de Costa Rica. Anales del Museo Nacional de Costa Rica 1888-1889: 45-64.
- Emery C. 1906. Studi sulle formiche della fauna neotropica. XXVI. Bullettino della Società Entomologica Italiana 37: 107-194.
- Escalante Gutiérrez J. A. 1993. Especies de hormigas conocidas del Perú (Hymenoptera: Formicidae). Revista Peruana de Entomología 34:1-13.
- Favretto M. A., E. Bortolon dos Santos, and C. J. Geuster. 2013. Entomofauna from West of Santa Catarina State, South of Brazil. EntomoBrasilis 6 (1): 42-63.
- Felizardo S. P. S., and A. Y. Harada. 2007. The genus Crematogaster Lund, 1831 (Formicidae: Myrmicinae: Crematogastrini) at ant collection from Emílio Goeli Paraense Museum (MPEG). Biológico, São Paulo 69(2): 425-427.
- Fernández F., E. E. Palacio, W. P. Mackay, and E. S. MacKay. 1996. Introducción al estudio de las hormigas (Hymenoptera: Formicidae) de Colombia. Pp. 349-412 in: Andrade M. G., G. Amat García, and F. Fernández. (eds.) 1996. Insectos de Colombia. Estudios escogidos. Bogotá: Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 541 pp
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Fisher B. L., L. da Silveira Lobo Sternber, and D. Price. 1990. Variation in the use of orchid extrafloral nectar by ants. Oecologia 83: 263-266.
- Forel A. 1897. Quelques Formicides de l'Antille de Grenada récoltés par M. H. H. Smith. Transactions of the Entomological Society of London. 1897: 297-300.
- Forel A. 1908. Ameisen aus Sao Paulo (Brasilien), Paraguay etc. gesammelt von Prof. Herm. v. Ihering, Dr. Lutz, Dr. Fiebrig, etc. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 58: 340-418.
- Forel A. 1908. Catálogo systemático da collecção de formigas do Ceará. Boletim do Museu Rocha 1(1): 62-69.
- Forel A. 1908. Fourmis de Costa-Rica récoltées par M. Paul Biolley. Bulletin de la Société Vaudoise des Sciences Naturelles 44: 35-72.
- Forel A. 1909. Ameisen aus Guatemala usw., Paraguay und Argentinien (Hym.). Deutsche Entomologische Zeitschrift 1909: 239-269.
- Forel A. 1912. Formicides néotropiques. Part III. 3me sous-famille Myrmicinae (suite). Genres Cremastogaster et Pheidole. Mémoires de la Société Entomologique de Belgique. 19: 211-237.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Herrera H.W., and L. Roque-Albelo. 2007. Lista anotada de las hormigas de las Islas Galapagos, Ecuador. Fundación Charles Darwin. 13 pp
- INBio Collection (via Gbif)
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Kusnezov N. 1963. Zoogeografia de las hormigas en sudamerica. Acta Zoologica Lilloana 19: 25-192
- Kusnezov N. 1978. Hormigas argentinas: clave para su identificación. Miscelánea. Instituto Miguel Lillo 61:1-147 + 28 pl.
- Larsen, A., and S. M. Philpott. 2010. Twig-nesting ants: the hidden predators of the coffee berry borer in Chiapas, Mexico. Biotropica 42: 342-347.
- Linsley E. G., and R. L. Usinger. 1966. Insects of the Galápagos Islands. Proc. Calif. Acad. Sci. (4) 33: 113-196.
- Longino J. et al. ADMAC project. Accessed on March 24th 2017 at https://sites.google.com/site/admacsite/
- Longino, J.T. 2003. The Crematogaster (Hymenoptera, Formicidae, Myrmicinae) of Costa Rica. Zootaxa 151:1-150
- Longino, J.T. 2010. Personal Communication. Longino Collection Database
- Luederwaldt H. 1918. Notas myrmecologicas. Rev. Mus. Paul. 10: 29-64.
- Lutinski J. A., F. R. Mello Garcia, C. J. Lutinska, and S. Iop. 2008. Ants diversity in Floresta Nacional de Chapecó in Santa Catarina State, Brazil. Ciência Rural, Santa Maria 38(7): 1810-1816.
- Maes, J.-M. and W.P. MacKay. 1993. Catalogo de las hormigas (Hymenoptera: Formicidae) de Nicaragua. Revista Nicaraguense de Entomologia 23.
- Mann W. M. 1916. The Stanford Expedition to Brazil, 1911, John C. Branner, Director. The ants of Brazil. Bulletin of the Museum of Comparative Zoology 60: 399-490
- Menozzi C. 1927. Formiche raccolte dal Sig. H. Schmidt nei dintorni di San José di Costa Rica. Entomologische Mitteilungen. Berlin-Dahlem. 16: 266-277.
- Menozzi C. 1935. Spedizione del Prof. Nello Beccari nella Guiana Britannica. Hymenoptera-Formicidae. Redia. 21: 189-203.
- Morgan C., and W. P. Mackay. 2017. The North America acrobat ants of the hyperdiverse genus Crematogaster. Mauritius: LAP LAMBERT Academic Publishing, 540 pp.
- Morgan, C.E. 2009. Revision of the ant genus Crematogaster (Hymenoptera: Formicidae) in North America. Ph.D. Dissertation, University of Texas at El Paso, 268 pp.
- Neves F. S., K. S. Queiroz-Dantas, W. D. da Rocha, and J. H. C. Delabie. 2013. Ants of Three Adjacent Habitats of a Transition Region Between the Cerrado and Caatinga Biomes: The Effects of Heterogeneity and Variation in Canopy Cover. Neotrop Entomol 42: 258268.
- Oliveira-Santos L. G. R., R. D. Loyola, A. B. Vargas. 2009. Canopy Traps: a Technique for Sampling Arboreal Ants in Forest Vertical Strata. Neotropical Entomology 38(5):691-694.
- Pergande T. 1894. Formicidae of Lower California, Mexico. Proc. Calif. Acad. Sci. (2) 4: 161-165.
- Philpott, S. M. 2006. Ant patchiness: a spatially quantitative test in coffee agroecosystems. Naturwissenschaften 93: 386-392.
- Pignalberi C. T. 1961. Contribución al conocimiento de los formícidos de la provincia de Santa Fé. Pp. 165-173 in: Comisión Investigación Científica; Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina) 1961. Actas y trabajos del primer Congreso Sudamericano de Zoología (La Plata, 12-24 octubre 1959). Tomo III. Buenos Aires: Librart, 276 pp.
- Radoszkowsky O. 1884. Fourmis de Cayenne Française. Trudy Russkago Entomologicheskago Obshchestva 18: 30-39.
- Reynoso-Campos J. J., J. A. Rodriguez-Garza, and M. Vasquez-Bolanos. 2015. Hormigas (Hymenoptera: Formicidae) de la Isla Cozumel, Quintana Roo, Mexico (pp. 27-39). En: Castaño Meneses G., M. Vásquez-Bolaños, J. L. Navarrete-Heredia, G. A. Quiroz-Rocha e I. Alcalá-Martínez (Coords.). Avances de Formicidae de México. UNAM, Universiad de Guadalajara, Guadalajara, Jalisco.
- Rico-Gray, V. 1993. Use of plant-derived food resources by ants in the dry tropical lowlands of coastal Veracruz, Mexico. Biotropica 25(3):301-315.
- Rico-Gray, V., and L. B. Thien. 1989. Ant-mealybug interaction decreases reproductive fitness of Schomburgkia tibicinis (Orchidaceae) in Mexico. Journal of Tropical Ecology 5: 109-112.
- Rico-Gray, V., and L. B. Thien. 1989. Effect of different ant species on reproductive fitness of Schomburgkia tibicinis (Orchidaceae). Oecologia 81: 487-489.
- Rico-Gray,V., J.G. Garcia-Franco, M. Palacios-Rios, C. Diaz-Castelazo, V. Parra-Tabla and J.A. Navarro. 1998. Geographical and Seasonal Variation in the Richness of Ant-Plant Interactions in Mexico. Biotropica 30(2):190-200.
- Rojas P., C. Fragoso, and W. P. MacKay. 2014. Ant communities along a gradient of plant succession in Mexican tropical coastal dunes. Sociobiology 61(2): 119-132.
- Rosa da Silva R. 1999. Formigas (Hymenoptera: Formicidae) do oeste de Santa Catarina: historico das coletas e lista atualizada das especies do Estado de Santa Catarina. Biotemas 12(2): 75-100.
- Santschi F. 1925. Nouveaux Formicides brésiliens et autres. Bulletin et Annales de la Société Entomologique de Belgique 65: 221-247.
- Tillberg, C.V. 2004. Cordia gerascanthus (Boraginaceae) Produces Stem Domatia. Journal of Tropical Ecology 20(3):355-357
- Vasquez-Bolanos M. 2011. Checklist of the ants (Hymenoptera: Formicidae) from Mexico. Dugesiana 18(1): 95-133.
- Vittar, F. 2008. Hormigas (Hymenoptera: Formicidae) de la Mesopotamia Argentina. INSUGEO Miscelania 17(2):447-466
- Vittar, F., and F. Cuezzo. "Hormigas (Hymenoptera: Formicidae) de la provincia de Santa Fe, Argentina." Revista de la Sociedad Entomológica Argentina (versión On-line ISSN 1851-7471) 67, no. 1-2 (2008).
- Vásquez-Bolaños M. 2011. Lista de especies de hormigas (Hymenoptera: Formicidae) para México. Dugesiana 18: 95-133
- Wetterer J.K. and J.L.W. Keularts. 2008. Population explosion of the hairy crazy ant, Paratrechina pubens (Hymenoptera: Formicidae), on St. Croix, US Virgin Islands. Florida Entomologist 91(3): 423-427.
- Wetterer, J.K. and J.L.W. Keularts. 2008. Population Explosion of the Hairy Crazy Ant, Paratrechina pubens (Hymenoptera: Formicidae), on St. Croix, US Virgin Islands. The Florida Entomologist 91(3):423-427
- Wheeler W. M. 1905. The ants of the Bahamas, with a list of the known West Indian species. Bulletin of the American Museum of Natural History 21: 79-135.
- Wheeler W. M. 1907. A collection of ants from British Honduras. Bulletin of the American Museum of Natural History 23: 271-277.
- Wheeler W. M. 1922. The ants of Trinidad. American Museum Novitates 45: 1-16.
- Wheeler W. M. 1925. Neotropical ants in the collections of the Royal Museum of Stockholm. Arkiv för Zoologi 17A(8): 1-55.
- Wheeler W. M. 1942. Studies of Neotropical ant-plants and their ants. Bulletin of the Museum of Comparative Zoology 90: 1-262.
- Wheeler, William Morton. 1911. Additions to the Ant-Fauna of Jamaica. Bulletin American Museum of Natural History. 30:21-29.
- Wheeler, William Morton. 1911. Ants Collected in Grenada, W.I. by Mr. C. T. Brues. Bulletin of the Museum of Comparitive Zoology at Harvard College. 54(5):166-172.
- Wheeler, William Morton. 1923. Report on the Ants. The University of Iowa Studies in Natural History. 10(3):3-9.
- Wheeler, William Morton. 1933. Formicidae of the Templeton Crocker Expedition. California Academy of Sciences. 21(6):57-64.
- Wild, A. L.. "A catalogue of the ants of Paraguay (Hymenoptera: Formicidae)." Zootaxa 1622 (2007): 1-55.
- Wilson, E.O. 1987. The Arboreal Ant Fauna of Peruvian Amazon Forests: A First Assessment. Biotropica 19(3):245-251.
- Yanoviak S. P., and M. Kaspari. 2000. Community structure and the habitat templet: ants in the tropical forest canopy and litter. Oikos 89: 259-266.
- Zolessi L. C. de, Y. P. Abenante, and M. E. de Philippi. 1988. Lista sistematica de las especies de Formicidos del Uruguay. Comun. Zool. Mus. Hist. Nat. Montev. 11: 1-9.
- Zolessi L. C. de; Y. P. de Abenante, and M. E. Philippi. 1989. Catálogo sistemático de las especies de Formícidos del Uruguay (Hymenoptera: Formicidae). Montevideo: ORCYT Unesco, 40 + ix pp.
- da Silva de Oliveira A. B., and F. A. Schmidt. 2019. Ant assemblages of Brazil nut trees Bertholletia excelsa in forest and pasture habitats in the Southwestern Brazilian Amazon. Biodiversity and Conservation 28(2): 329-344.
- de Zolessi, L.C., Y.P. de Abenante and M.E. Philippi. 1987. Lista sistemática de las especies de formícidos del Uruguay. Comunicaciones Zoologicas del Museo de Historia Natural de Montevideo 11(165):1-9
- de Zolessi, L.C., Y.P. de Abenante and M.E. Phillipi. 1989. Catalago Systematico de las Especies de Formicidos del Uruguay (Hymenoptera: Formicidae). Oficina Regional de Ciencia y Technologia de la Unesco para America Latina y el Caribe- ORCYT. Montevideo, Uruguay
- Pages using DynamicPageList3 parser function
- North temperate
- North subtropical
- Tropical
- South subtropical
- South temperate
- Eulophid wasp Associate
- Host of Melittobia australica
- Syrphid fly Associate
- Host of Stipomorpha wheeleri
- Species
- Extant species
- Formicidae
- Myrmicinae
- Crematogastrini
- Crematogaster
- Crematogaster crinosa
- Myrmicinae species
- Crematogastrini species
- Crematogaster species
- Ssr

