Aneuretus simoni
| Aneuretus simoni | |
|---|---|

| |
| Conservation status | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Aneuretinae |
| Genus: | Aneuretus |
| Species: | A. simoni |
| Binomial name | |
| Aneuretus simoni Emery, 1893 | |
| Synonyms | |
| |
Aneuretus simoni is the only species of this genus and is the only extant member of the subfamily Aneuretinae. Long believed to be one of the basal lineages of Formicidae, molecular phylogenetics have shown the subfamily is closely related to the Dolichoderinae (see the subfamily phylogeny).
| At a Glance | • Polygynous |
Identification
Sting (that may not be visible), single petiole with a long, narrow anterior peduncle, propodeum armed with a pair of spines. Major tibial spur of hind leg simple or with a few minute barbules. Palp forumula 3,4. Only known from Sri Lanka.
In the field workers resemble small yellow Pheidole minors in their morphology. Behaviorally they have a tendency to keep their long petiole folded up against their propodeum with their gaster slightly elevated.
Distribution
As reported in Dias et al (2011): A. simoni has been recorded from “Udawatta Kele” and Peradeniya in Kandy District and “Pompekelle”—a disturbed forest—and Gilimale and Adam’s Peak Forest Reserves in Ratnapura District (Wilson et al. 1956), but in 1979, its presence was confirmed only from Gilimale Forest Reserve (Jayasuriya & Traniello 1985). Recently, A. simoni has been recorded from “Pompekelle” (Chaminda & Dias 2001; Dias 2004) and from Gilimale Forest Reserve (Dias 2008), and from the Mulawella region of Sinharaja Forest Reserve, which is located in Ratnapura District, from 2005 (Perera et al. 2006; Dias 2008) to February 2007 (Gunawardene et al. 2008). Sinharaja Forest Reserve, a World Heritage site, lies in three districts—Ratnapura, Kalutara and Matara—in the three provinces: Sabaragamuwa, Western and Southern, respectively (Gunawardene 2003). We discovered a group of A. simoni workers (collector: H.P.G.R.C. Ruchirani) in December 2009 and two colonies of the species in January 2010 from the floor of a wet evergreen rainforest, “Kirikanda” (06°25´N, 80°20´E; elevation 112 m), which lies very close to the Sinharaja boundary in Kalutara District.
Latitudinal Distribution Pattern
Latitudinal Range: 8.481666667° to 6.099722222°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Oriental Region: Sri Lanka (type locality).
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
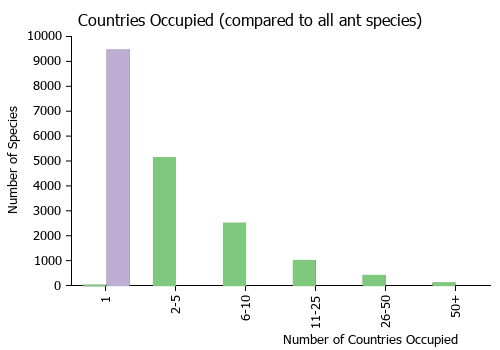
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |
|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Habitat
Aneuretus have been collected in primary rainforest and forests with dense secondary growth. Both forest types are extremely humid, with the secondary growth regions were thickly carpeted with leaf litter. The predominant tree genera in the forests where A. simoni have been studied are Bombax, Dipterocarpus, CaUophyUum, Myristica, Artocarpus, Syzygium, Doona, and Mesua (Jayasuriya and Traniello 1986). Wilson et. al (1956) noted colonies were most abundant at the edge of clearings.
Biology
A recent survey (Dias and Udayakantha 2016) reported: Nests of A. simoni were found in only one locality. Higher frequency of occurrence (4/40 to 8/40) than observed for other species was observed for A. simoni in all samples except in June, and the percentage nest abundance ranged from 5.7 to 20.5. The mean nest density of the species was among the highest four on each occasion and was, along with a Pheidole species, significantly higher overall than that of 14 other species. The nest density of A. simoni ranged from 0.2 - 0.8 m-2 from March to July in the forest, and this was higher than that recorded at its other habitats except the Kuluna Kanda Proposed Forest Reserve. The mean nest density of A. simoni had no significant correlation with monthly rainfall. Aneuretus simoni is locally-dominant at an altitude of 57 m in Meethirigala Forest Reserve, which is the lowest elevation record for the species.
The following information is based on and includes excerpts from Jayasuriya and Traniello (1986) and Wilson et. al (1956).
Foraging/Diet
In the field (as in the laboratory), Aneuretus appears to be a scavenger, and workers feed on nectar and prey upon small insects. The sting is used to paralyze prey. Nectar from fallen fruits and flowers is readily imbibed. Several Aneuretus nests contained coccids and Aneuretus may feed on the sap extruded by these insects. Leaf litter samples taken around their nests contained abundant small insects (entomobryoid collembolans, and cerambycid and carabid larvze). Many of these litter organisms are likely to be potential prey of Aneuretus, and constitute the protein component of its diet.
Larvae feed directly upon prey delivered to them by workers. Adult trophallaxis has been observed in the lab.
Workers forage in and on the leaf litter, favor shaded areas, and in foliage up to 1 1/3 m above ground. Odor trails are used to recruit nestmates to food. Workers appear to forage preferentially at low temperatures, and colonies are active in the morning and late afternoon, ceasing foraging at noon.
Foraging workers move at about an average gait for ants of comparable size; very roughly speaking, they are slower than most dolichoderines, such as Technomyrmex albipes, but faster than most ponerines, such as Ponera coarctata. Like many of the higher dolichoderines, they are much given to halting abruptly at intervals, standing motionless for a moment, and then abruptly starting off again, often in another direction. When they are disturbed away from the nest, this behavior is intensified, with the workers dashing for a short distance, then halting and remaining' perfectly still for a while, then dashing off again; under these conditions they show themselves capable of considerable speed and agility.
Colony Attributes
Aneuretus colonies can contain multiple queens and have approximately 65 minor workers. Major workers comprise only two to three percent of the colony population. Sexuals are found in nests from mid-July to August.
Nesting Habits
Aneuretus nests in pieces of rotting wood on the ground, in fallen branches from twig size up to 75mm in diameter. Many nests were located in the hollow core (about 0.5 cm in diameter) of small twigs, a few were found under the bark of rotting logs, and one was under a layer a leaf litter.
Colonies appear to be polydomous. In the field, a mature colony is usually divided into two or three neighboring groups. The chambers, which comprise the subunits of a nest, are not necessarily connected by galleries, but worker traffic on the surface of the ground is often seen between subnests, and can involve the transfer of brood and queens. In the laboratory, approximately half of one colony moved into a second nest tube, even though adequate space for the entire colony was available in any single tube.
Reproduction
Alates were found in nests collected after mid-July. July and August are the dryest months in the Gilimale region followed by monsoons in October. Assuming alates are released before October, sexuals are likely produced during the dry season under conditions favorable for dispersal, but at a time shortly preceding the monsoon, to insure sufficient moisture during colony foundation.
Behavior
A. simoni workers avoid contact with workers of sympatric ant genera such as Pheidole, Paratrechina, Tetramorium, Odontomachus, and Monomorium. When alien conspecifics are encountered, aggression results, indicating that nestmate recognition and perhaps intraspecific territoriality is well developed. Non-nestmates placed into a foreign nest are readily attacked, with the otherwise timid workers engaging in combat for hours, biting and stinging each other.
When a nest is first broken open, the minor workers swarm out aggressively and run over any alien object offered them, but they do not attempt to bite or sting. Major workers seem to be more timid, and do not venture out in this fashion. The aggressiveness of the minor workers is usually short-lived, and they soon retreat back into the undisturbed part of the nest, leaving unprotected any brood which may happen to be in the exposed chambers. Later, they venture out singly to retrieve the brood.
An ethology study (Traniello and Jayasuriya 1986) found: "Observed repertory sizes were 5 acts (the queen), 14 acts (major workers), 28 acts (callow minor workers) and 31 acts (mature minor workers). These repertory sizes are comparable to those observed in other ant species. Major workers, which average less than two in number per colony, do not show brood care. Majors were also never observed to participate in colony defense. The behavioral repertory of callow minor workers includes queen-related acts, brood care, and foraging. A comparison of social organization in A. simoni and other so-called primitive and advanced species indicates that social behavior is very similar to that of dolichoderine species."
Chemistry
Traniello and Jayasuriya (1981): Trail and alarm communication in Aneuretus simoni are mediated by the secretions of the sternal and pygidial glands, respectively. The sternal gland is composed of a glandular epithelium and an associated reservoir located in the 7th sternum. This gland produces a relatively long-lived mass recruitment pheromone. The pygidial gland opens between the 6th and 7th tergites and produces a secretion that releases aggressive alarm.
Conservation Status
This species is on the IUCN Red List of Threatened Species.
Flight Period
| X | X | ||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
- Check details at Worldwide Ant Nuptial Flights Data, AntNupTracker and AntKeeping.
- Explore: Show all Flight Month data or Search these data. See also a list of all data tables or learn how data is managed.
Life History Traits
- Queen number: polygynous (Rissing and Pollock, 1988; Frumhoff & Ward, 1992)
- Mean colony size: 65-75 (Traniello & Jayasuriya, 1981; Jayasuria & Traniello, 1985; Greer et al., 2021)
- Compound colony type: not parasitic (Greer et al., 2021)
- Nest site: hypogaeic (Greer et al., 2021)
- Diet class: omnivore (Greer et al., 2021)
- Foraging stratum: subterranean/leaf litter (Greer et al., 2021)
- Foraging behaviour: cooperative/mass recruiter (Traniello & Jayasuriya, 1981; Jayasuria & Traniello, 1985; Beckers et al., 1989; Greer et al., 2021)
Morphology
Exocrine system
Billen (2017b) - In comparison with their stingless sister group Dolichoderinae, the stinging A. simoni has a well-developed venom gland with long and slender secretory filaments. They also share the occurrence of Pavan’s gland as source of the trail pheromone, although this gland has a bilobed appearance in Aneuretus, while it only has one lobe in dolichoderine species. The intramandibular gland is well-developed, while the metapleural gland has the lowest number of secretory cells known in ant workers. All glands described occur in both minor and major workers, with sometimes clear differences between both worker castes. Major workers have larger propharyngeal glands, which can be understood by their presumed role in food storage in the colony, and also have metapleural glands with twice as many secretory cells than minor workers. Minor workers in turn have a more developed venom gland, which is in line with their higher activity in nest defence and prey capture.
Intramandibular gland
Billen and Verbesselt (2016) - Aneuretus simoni workers have a conspicuous intramandibular gland, formed by round to polygonal class-3 secretory cells with a diameter around 16-17 μm and their accompanying duct cells. Both in minor and major workers, approx. 8 cells open through the proximal upper surface of the mandible, approx. 12 cells open through the proximal lower surface. The secretory cells are characterized by a well-developed granular endoplasmic reticulum and Golgi apparatus. The cytoplasmic organization indicates the secretion has a proteinaceous nature, and therefore probably does
Metapleural gland
Billen (2017a) - Workers have a paired metapleural gland that in minor workers contains six secretory cells on each side, while majors have 12 cells per side. The heavily sclerotized reservoir opens above the articulation of the hind-legs through a large round orifice with a diameter around 40 μm. Stiff dispenser hairs emerge from the orifice, and assist in guiding the secretory products to the exterior. The rounded to ovoid cells display a well-developed smooth endoplasmatic reticulum, which is a common characteristic for the metapleural gland in ants. This cytoplasmic organization is in agreement with the production of antibiotic substances, which is the most common function of this gland. The difference in gland size between minor and major workers may either indicate that majors are more efficient in producing antibiotics, or that the gland may serve another function, as the cell numbers in A. simoni are the lowest known of all ant species studied so far.
Pavan's gland
Billen and Verbesselt (2016b) - Workers of Aneuretus simoni have a well-developed Pavan’s gland, which is the exocrine character that makes the Aneuretinae and Dolichoderinae sister groups. The secretory epithelium of the gland consists in both minor and major workers of an anterior and a posterior part, which makes them different from the Dolichoderinae where the gland is formed by a single part only. At the ultrastructural level, the secretory cells are characterized by a very prominent smooth endoplasmic reticulum, which is in line with the production of trail pheromones in this species. The presence of the gland and hence the ability to lay trail pheromones can be understood as literature data report trail involving behaviours in both castes, minor workers being more active in foraging, while majors play a role during nest emigration.
Castes
The single extant species of the genus has dimorphic workers (see illustration in next section). Wilson et al (1956): "the major worker differs from the minor primarily in its proportionately larger and broader head, and in its relatively shorter, stouter propodeal spines. In the queen, it will be noted both of these allometric trends are carried a bit further. The head is wider with respect to its length than in the worker castes, and the propodeal spines are reduced to mere angles."
Worker
Images from AntWeb
   
| |
| Worker. Specimen code casent0010853. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by UCDC, Davis, CA, USA. |
   
| |
| Worker. Specimen code casent0102369. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
   
| |
| Worker. Specimen code casent0102370. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
   
| |
| Worker. Specimen code casent0172258. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ANIC, Canberra, Australia. |
Queen
Images from AntWeb
   
| |
| Queen (alate/dealate). Specimen code casent0172259. Photographer April Nobile, uploaded by California Academy of Sciences. | Owned by ANIC, Canberra, Australia. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- simoni. Aneuretus simoni Emery, 1893a: cclxxvi (w.) SRI LANKA.
- Type-material: 2 syntype workers.
- Type-locality: Sri Lanka (“Ceylon”): Kandy, i.-ii.1892 (E. Simon).
- Type-depository: MSNG.
- [Also described as new by Emery, 1893f: 242.]
- Wilson, et al. 1956: 85 (q.m.l.).
- Status as species: Emery, 1893f: 242; Forel, 1895e: 462; Bingham, 1903: 291; Emery, 1913a: 7; Chapman & Capco, 1951: 181; Wilson, et al. 1956: 82 (redescription); Gotwald, 1970: 950; Jayasuriya & Traniello, 1986: 363; Traniello & Jayasuriya, 1986: 375; Shattuck, 1994: 1; Bolton, 1995b: 63; Billen, 2017: 1.
- Senior synonym of butteli: Wilson, et al. 1956: 81 (footnote); Shattuck, 1994: 1; Bolton, 1995b: 63.
- Distribution: Sri Lanka.
- butteli. Aneuretus butteli Forel, 1912m: 770 (q.) SRI LANKA.
- Type-material: holotype queen.
- Type-locality: Sri Lanka (“Ceylon”): (no further data) (von Buttel-Reepen).
- Type-depository: MHNG.
- [Also described as new by Forel, 1913k: 87.]
- Status as species: Chapman & Capco, 1951: 181.
- Junior synonym of simoni: Wilson, et al. 1956: 81 (footnote); Shattuck, 1994: 1; Bolton, 1995b: 63.
Description
From Wilson et al. (1956):
Worker
The following description is based on the minor worker. Eyes small, containing only about 30 ommatidia. Antennae 12-segmented, with the segments of the flagellum increasing distally both in length and width. Clypus broad and flat, lacking a median carina, the anterior border strongly emarginate in fullface view. Mandible as shown in Plate 2B. Mandibular dentition reminiscent of Dolichoderus (s. str.), relatively constant except in the following two charaeters: (1) the shapes of all the individual teeth vary somewhat, and (2) the number of the reduced median teeth (located between the three apical and three major basal teeth) varies from four to right. Maxillary palp 3-segmented, the terminal segment showing a variabile median constriction or local cuticular thickening, which may well indicate a phylogenetically recent fusion site. Labial palp 4 - segmented.
Alitrunk as figured. Propodeal spines spike-like, tapering abruptly near the tips to form sharp points; seen from above, they diverge from one another strongly. Metasternal gland bulla prominent, its openings supplied with a small number of coarse guard hairs originating from both the dorsal and ventral lips. Anterior peduncle of petiole long and slender, somewhat narrowed toward its anterior attachment. The petiolar node well differentiated from the anterior peduncle by dorsal and lateral swellings. Seen from above, the node proper is nearly twice as broad as long. Posteriorly, it narrows abruptly to the point of attachment to the gaster.
The gaster lacks any form of intersegmental constriction whatsoever, The sting is well developed, sclerotized, and exsertile.
Integument relatively thin and collapsible, resembling in this respect that of the higher dolichoderines and formicines. Color of living and fresh alcoholic material varies within most nest series from light yellow (in callows) to medium yellowish orange. Body lightly shagreened overall, subopaque to feebly shining; the dorsal and most of the lateral surfaces of the propodeum are in addition transversely rugulose.
Pilosity generally sparse. Appendages covered with abundant short, oblique pubesence but lacking longer hairs except on the coxae and tarsal claws. Body supplied with scattered, relatively long, pointed erect hairs distributed as shown in Plate 1; body pubeseence appressed and sparse.
Queen
Queen. (Plate 1.) The queen is much larger in size than either of the worker subcastes and differs further in its proportionately broader head and greatly reduced propodeal spines. In addition, it is much darker, being overall dark brown. It is essentially similar to the workers in petiolar structure, mandibular dentition, pilosity, sculpturing, etc.
The wing venation (of both the queen and the male) is relatively generalized and similar to that of the most generalized true dolichoderine, Dolichoderus s. str., as typified by [Dolichoderus attelaboides] (Fabr.) . The latter species, as pointed out by Brown and Nutting (1950) in their important review of formicid wing venation, possesses all of the venational elements of the primitive ponerine pattern except for the first radial cross-vein. Aneuretus simoni shows two significant further advances. First, Mf2 is completely contracted, so that the ends of Rs2 and the cross-vein m-cu are contiguous. Second, Rs4 has contracted to bring about the alignment of the cross-veins 2r and r-m.
Male
Plates 1 and 2 show almost all of the important features of external morphology of this castre Especially noteworthy are the conservation of the" myrmecioid" petiolar structure shown also by the worker and queen, the relatively generalized structure of the mandible, and the generalized structure of the genitalia, especially the volsella. One perhaps significant advanced feature is the absence of serration on the ventral edge of the penis valve.
Larva
Immature larva. (Text-fig. 3.) Body length (through spiracles) 1.1-2.1 mm. Straight length 0.8-1.6 mm. Somewhat sigmoidal in sideview; thorax forming a stout neck which is bent ventrally at 90 degrees; abdomen rather stout, diameter greatest at abdominal somite IV; abdominal somites IX and X small and directed posterodorsally, the tenth forming a terminal naked knob. Anus posterior. Leg and wing rudiments present. Spiracles small, decreasing in diameter posteriorly. No spinules seen on the integument. Body hairs short, moderately numerous, uniformly distributed, of three types: (l) 0.027-0.045 mm. long, slightly curved or flexous, with a few minute denticles, without alveolus and articular membrane, the most numerous type; (2) O.045-0.0n mm. long, longest and most numerous anteriorly, slightly curved or flexuous, with a few minute denticles, with alveolus and articular membrane; (3) minute (about 0.001 mm. long), few, widely scattered. Head moderately large, feebly cordate; cranium transversely subelliptical, with the occipital border impressed and the corners rounded. Mouth parts large. Antennae small, each with three sensilla, each of which bears a moderately long spinule. Head hairs moderately numerous, uniformly distributed, slightly curved or flexuous, long (0.027-0.072 mm.), with numerous minute denticles on the distal 2/3. Labrum short and broad (breadth 2.8 times the length), bilobed (due to a rather deep impression of the ventral border; anterior surface of each lobe with one minute hair and three sensilla; ventral border with two sensilla near the middle and with a few minute spinules; posterior surface of each lobe with one large and three minute sensilla; posterior surface spinulose, the spinules minute and in numerous short arcuate rows, the rows radiating from each dorsolateral corner. Mandibles moderately large, heavily sclerotized and subtriangular in anterior view, longer than broad; slightly curved posteriorly; apex forming a rather long tooth which is slightly curved medially, two rather large subapical teeth on the medial border; anterior surface with numerous short longitudinal ridges (rows of exceedingly minute spinules); posterior surface with several acute denticles on the distal half and numerous ridges on the basal half, some of the ridges bearing a row of minute spinules. Maxillae lobose, with the apex a small conoid directed medially, with minute spinu1es in short rows which are arranged in longer rows encircling the galea; palp a frustum bearing four apical (two encapsulatrd and two with a spinule each) and one lateral (with a spinule) sensilla; galea a tall peg with two apical sensilla. Labium hemispheroidal and protruding; with all surfaces spinulose; the spinules more numerous and in more numerous rows on the anterior surface, and larger, fewer, and in short rows or isolated on the posterior surface; palp a slight elevation with five sensilla, three encapsulated and two bearing a spinule each. Opening of sericteries wide and salient. Hypopharynx spinulose, the spinnles so long that those in adjacent rows overlap; the rows numerous and radiating from each dorsolateral angle.
The larva of Aneuretus shows only one dolichoderine character --the short, broad labrum -- and even this is not distinctive. The one and only character that affiliates it with the Dolichoderinae is, strangely enough, one which is not characteristic of the subfamily at all, namely the terminal knob projecting posteriorly or posterodorsally. Now, a terminal knob occurs in the larvae of several genera in other subfamilies, but there it projects posteroventrally, ventrally, or anteroventrally. Only in the most advanced dolichoderine genera Engramma, Tapinoma and Technomyrmex have we previously found it projecting posteriorly or posterodorsally. (See G. C. and .J. Wheeler, 1951.)
In marked contrast are the many non-dolichoderine characters of Aneuretus larvae. These are given in the accompanying table (see Wilson et al. 1956). It will be seen that all the non-dolichoderine characters of Aneuretus except 3 and 4 are more generalized than their dolichoderine counterparts. Thus we find Aneuretus standing out conspicuously as a generalized ant larva when considered in reference to the Dolichoderinae.
References
- Barden, P. 2017. Fossil ants (Hymenoptera: Formicidae): ancient diversity and the rise of modern lineages. Myrmecological News 24: 1-30.
- Beckers R., Goss, S., Deneubourg, J.L., Pasteels, J.M. 1989. Colony size, communication and ant foraging Strategy. Psyche 96: 239-256 (doi:10.1155/1989/94279).
- Billen, J. and S. Verbesselt. 2016a. The intramandibular gland of Aneuretus simoni (Formicidae, Aneuretinae). Asian Myrmecology. 8:95-99. doi:10.20362/am.008009
- Billen, J. and S. Verbesselt. 2016b. Morphology and ultrastructure of Pavan’s gland of Aneuretus simoni (Formicidae, Aneuretinae). Asian Myrmecology. 8:101-106. doi:10.20362/am.008017
- Billen, Johan. 2017a. The metapleural gland of Aneuretus simoni (Formicidae, Aneuretinae). Asian Myrmecology 9: e00905 (1-5). doi:10.20362/am.009005
- Billen, Johan. 2017b. The exocrine system of Aneuretus simoni (Formicidae, Aneuretinae). Asian Myrmecology 9: e009011 (1-16), doi:10.20362/am.009011
- Borowiec, M.L., Moreau, C.S., Rabeling, C. 2020. Ants: Phylogeny and Classification. In: C. Starr (ed.), Encyclopedia of Social Insects (doi:10.1007/978-3-319-90306-4_155-1).
- Boudinot, B.E., Richter, A.K., Hammel, J.U., Szwedo, J., Bojarski, B., Perrichot, V. 2022. Genomic-phenomic reciprocal illumination: Desyopone hereon gen. et sp. nov., an exceptional Aneuretine-like fossil ant from Ethiopian amber (Hymenoptera: Formicidae: Ponerinae). Insects 13(9), 796 (doi:10.3390/insects13090796).
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Cantone S. 2018. Winged Ants, The queen. Dichotomous key to genera of winged female ants in the World. The Wings of Ants: morphological and systematic relationships (self-published).
- Dias R.K.S. 2020. Aneuretus simoni. In: Starr C. (eds) Encyclopedia of Social Insects (doi:10.1007/978-3-319-90306-4_6-1).
- Dias, R. K. S. and W. S. Udayakantha. 2016. Discovery of the Sri Lankan Relict Ant, Aneuretus simoni Emery (Formicidae, Aneuretinae) and the nest density of the species in a selected region of Meethirigala Forest Reserve, Sri Lanka. Asian Myrmecol. 8:49-56. doi:10.20362/am.008005
- Dias, R.K.S. & Perera, K.A.M. 2011. Worker ant community observed by repeated sampling and information on endemic Aneuretus simoni Emery in the Gilimale Forest Reserve in Sri Lanka. Asian Myrmecology, 4, 69–78.
- Dias, R.K.S., Peiris, H.A.W.S. & Ruchchirani, H.P.G.R.C. 2011. Discovery of Aneuretus simoni Emery in a disturbed forest in Kalutara, and Stereomyrmex horni Emery in Anuradhapura Sanctuary, Sri Lanka. Asian Myrmecology. 4:99-102. PDF
- Dias, R.K.S., Rajapaksa, R.P.K.C. 2017. Geographic records of subfamilies, genera and species of ants (Hymenoptera: Formicidae) in the four climatic zones of Sri Lanka: A review. Journal of Science of the University of Kelaniya Sri Lanka 11, 23-45. (doi:10.4038/josuk.v11i2.7999).
- Dias, R.K.S., Ruchirani, H.P.G.R.C. 2014. Nest density of Aneuretus simoni Emery (Sri Lankan Relict Ant) and Stereomyrmex horni Emery in three forest regions in western and southern Sri Lanka. Asian Myrmecology 6, 83-90
- Dias, R.K.S., Ruchirani, H.P.G.R.C., Kosgamage, K.R.K.A. & Peiris, H.A.W.S. 2013. Frequency of nest occurrence and nest density of Aneuretus simoni Emery (Sri Lankan Relict Ant) and other ant fauna in an abandoned rubber plantation (Kirikanda Forest) in southwest Sri Lanka. Asian Myrmecology 5, 59–67.
- Emery, C. 1893a [1892]. [Untitled. Introduced by: "M. C. Emery, de Bologne, envoie les diagnoses de cinq nouveaux genres de Formicides".]. Bull. Bimens. Soc. Entomol. Fr. 1892:cclxxv-cclxxvii. (page cclxxvi, worker described)
- Emery, C. 1893h. Voyage de M. E. Simon à l'île de Ceylan (janvier-février 1892). Formicides. Ann. Soc. Entomol. Fr. 62: 239-258 (page 242, also described as new)
- Fernandez, F., Guerrero, R.J., Sánchez-Restrepo, A.F. 2021. Sistemática y diversidad de las hormigas neotropicales. Revista Colombiana de Entomología 47, 1–20 (doi:10.25100/socolen.v47i1.11082).
- Gotwald, W. H., Jr. 1970. Mouthpart morphology of the ant Aneuretus simoni. Ann. Entomol. Soc. Am. 63: 950-952 (page 950, see also)
- Hermann, H. R. 1968. The hymenopterous poison apparatus. V. Aneuretus simoni. Annals of the Entomological Society of America. 61:1315-1317.
- Jayasuriya, A. K. and J. F. A. Traniello. 1986. The biology of the primitive ant Aneuretus simoni (Emery) (Formicidae: Aneuretinae). I. Distribution, abundance, colony structure, and foraging ecology. Insectes Sociaux. 32:363-374.
- Meurville, M.-P., LeBoeuf, A.C. 2021. Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae). Myrmecological News 31: 1-30 (doi:10.25849/MYRMECOL.NEWS_031:001).
- Radchenko, A. 2023. A new extinct ant genus (Hymenoptera: Formicidae) from Baltic Amber. Annales Zoologici 73(1), 41-49 (doi:10.3161/00034541anz2023.73.1.004).
- Radchenko, A., Khomych, M. 2022. First records of aneuretine ants (Hymenoptera: Formicidae: Aneuretinae) in late Eocene Rovno amber (Ukraine). Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa” 65 (2): 69–80 (doi:10.3897/travaux.65.e85206).
- Shattuck, S. O. 1994. Taxonomic catalog of the ant subfamilies Aneuretinae and Dolichoderinae (Hymenoptera: Formicidae). Univ. Calif. Publ. Entomol. 112:i-xix, 1-241. (page 1, see also)
- Traniello, J.F.A. & Jayasuriya, A.K. 1981. Chemical communication in the primitive ant Aneuretus simoni: the role of the sternal and pygidial glands," Journal of Chemical Ecology. 7:1023-1033.
- Traniello, J.F.A.; Jayasuriya, A.K. 1986 [1985]. The biology of the primitive ant Aneuretus simoni (Emery) (Formicidae: Aneuretinae). II. The social ethogram and division of labor. Insectes Soc. 32: 375-388 (page 375, see also)
- Udayakantha, W.S., Dias, R.K.S., Rajapakse, R.P.K.C. 2023. Geographical records of six common ant species (Hymenoptera: Formicidae) in three climatic zones of Sri Lanka. Caucasian Entomological Bulletin 19(1), 71–78 (doi:10.23885/181433262023191-7178).
- Wilson, E. O., T. Eisner, G. C. Wheeler, and J. Wheeler. 1956. Aneuretus simoni Emery, a major link in ant evolution. Bulletin of the Museum of Comparative Zoology. 115:81-99. (page 82, queen, male, larva described)
References based on Global Ant Biodiversity Informatics
- CSIRO Collection
- Chapman, J. W., and Capco, S. R. 1951. Check list of the ants (Hymenoptera: Formicidae) of Asia. Monogr. Inst. Sci. Technol. Manila 1: 1-327
- Dias R. K. S. 2002. Current knowledge on ants of Sri Lanka. ANeT Newsletter 4: 17- 21.
- Dias R. K. S. 2006. Current taxonomic status of ants (Hymenoptera: Formicidae) in Sri Lanka. The Fauna of Sri Lanka: 43-52. Bambaradeniya, C.N.B. (Editor), 2006. Fauna of Sri Lanka: Status of Taxonomy, Research and Conservation. The World Conservation Union, Colombo, Sri Lanka & Government of Sri Lanka. viii + 308pp.
- Dias R. K. S. 2013. Diversity and importance of soil-dweeling ants. Proceedings of the National Symposium on Soil Biodiversity, chapt 4, pp 19-22.
- Dias R. K. S., H. P. G. R. C. Ruchirani, K. R. K. A. Kosgamage, and H. A. W. S. Peiris. 2013. Frequency of nest occurrence and nest density of Aneuretus simoni Emery (Sri Lankan Relict Ant) and other ant fauna in an abandoned rubber plantation (Kirikanda Forest) in southwest Sri Lanka. Asian Myrmecology 5: 59-67.
- Dias R. K. S., K. R. K. A. Kosgamage, and H. A. W. S. Peiris. 2012. The Taxonomy and Conservation Status of Ants (Order: Hymenoptera, Family: Formicidae) in Sri Lanka. In: The National Red List 2012 of Sri Lanka; Conservation Status of the Fauna and Flora. Weerakoon, D.K. & S. Wijesundara Eds., Ministry of Environment, Colombo, Sri Lanka. p11-19.
- Dias R. K. S., and H. P. G. R. C. Ruchirani. 2014. Nest density of Aneuretus simoni Emery (Sri Lankan Relict Ant) and Stereomyrmex horni Emery in three forest regions in western and southern Sri Lanka. Asian Myrmecology 6: 83-90.
- Dias R. K. S., and R. P. K. C. Rajapaksa. 2016. Geographic records of subfamilies, genera and species of ants (Hymenoptera: Formicidae) in the four climatic zones of Sri Lanka: a review. J. Sci. Univ. Kelaniya 11(2): 23-45.
- Dias R. K. S., and W. S. Udayakantha. 2016. Discovery of the Sri Lankan Relict Ant, Aneuretus simoni Emery (Formicidae, Aneuretinae) and the nest density of the species in a selected region of Meethirigala Forest Reserve, Sri Lanka. Asian Myrmecology 8: 1-8. DOI: 10.20362/am.008005
- Dias R. K. S.; Peiris, H. A. W. S.; Ruchirani, H. P. G. R. C. 2011. Discovery of Aneuretus simoni Emery in a disturbed forest in Kalutara, and Stereomyrmex horni Emery in Anuradhapura Sanctuary, Sri Lanka. Asian Myrmecology 4: 99-102.
- Dias R. K. S.; Perera K. A. M. 2011. Worker ant community observed by repeated sampling and information on endemic Aneuretus simoni Emery in the Gilimale Forest Reserve in Sri Lanka. Asian Myrmecology 4: 69-78.
- Dias, R.K.S. 2006. Overview of ant research in Sri Lanka: 2000-2004. ANeT Newsletter 8:7-10
- Emery C. 1893. Voyage de M. E. Simon à l'île de Ceylan (janvier-février 1892). Formicides. Annales de la Société Entomologique de France 62: 239-258.
- Emery C. 1893. [Untitled. Introduced by: "M. C. Emery, de Bologne, envoie les diagnoses de cinq nouveaux genres de Formicides".]. Bulletin Bimensuel de la Société Entomologique de France 1892: cclxxv-cclxxvii.
- Emery C. 1913. Hymenoptera. Fam. Formicidae. Subfam. Dolichoderinae. Genera Insectorum 137: 1-50.
- Forel A. 1895. Les Formicides de l'Empire des Indes et de Ceylan. Part V. J. Bombay Nat. Hist. Soc. 9: 453-472.
- Forel A. 1912. Descriptions provisoires de genres, sous-genres, et espèces de Formicides des Indes orientales. Rev. Suisse Zool. 20: 761-774.
- Forel A. 1912. Descriptions provisoires de genres, sous-genres, et espèces de Formicides des Indes orientales. Revue Suisse de Zoologie 20: 761-774.
- Forel A. 1913k. Wissenschaftliche Ergebnisse einer Forschungsreise nach Ostindien ausgeführt im Auftrage der Kgl. Preuss. Akademie der Wissenschaften zu Berlin von H. v. Buttel-Reepen. II. Ameisen aus Sumatra, Java, Malacca und Ceylon. Gesammelt von Herrn Prof. Dr. v. Buttel-Reepen in den Jahren 1911-1912. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 36:1-148.
- Gumawardene, N.R., J.D. Majer and J.P. Edirisinghe. 2008. Diversity and richness of ant species in a lowland wet forest reserve in Sri Lanka. Asian Myrmecology 2:71-83
- Gunawardene N. R., J. D. Majer, and J. P. Edirisinghe. 2008. Diversity and richness of ant species in a lowland wet forest reserve in Sri Lanka. Asian Myrmecology 2: 71-83.
- Gunawardene N. R., J. D. Majer, and J. P. Edirisinghe. 2012. Correlates of ant 5Hymenoptera: Formicidae) and tree species diversity in Sri Lanka. Myrmecological News 17: 81-90.
- Jayasuriya A. K.; Traniello, J. F. A. 1986. The biology of the primitive ant Aneuretus simoni (Emery) (Formicidae: Aneuretinae). I. Distribution, abundance, colony structure, and foraging ecology. Insectes Sociaux 32:363-374.
- Karunarathna D. A. G. N. B., and W. A. I. P. Karunaratne. 2013. Two new localities of Sri Lankan Relict Ant Aneuretus simoni Emery, 1893 (Formicidae: Aneuretinae) with the very first record in the intermediate zone. Journal of Threatened Taxa 5(11): 4604-4607.
- Shattuck S. O. 1994. Taxonomic catalog of the ant subfamilies Aneuretinae and Dolichoderinae (Hymenoptera: Formicidae). University of California Publications in Entomology 112: i-xix, 1-241.
- Traniello J. F. A., and A. K. Jayasuriya. 1986. The biology of the primitive ant Aneuretus simoni (Emery). 2. The social ethogram and division of labor. Insectes Sociaux 32 (1985): 375-388.
- Wilson E. O., T. Eisner, G. C. Wheeler, and J. Wheeler. 1956. Aneuretus simoni Emery, a major link in ant evolution. Bulletin of the Museum of Comparative Zoology 115: 81-99.

















