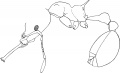
Acanthognathus rudis
| Acanthognathus rudis | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Acanthognathus |
| Species: | A. rudis |
| Binomial name | |
| Acanthognathus rudis Brown & Kempf, 1969 | |
| At a Glance | • Ergatoid queen |
Identification
Keys including this Species
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: -21.969° to -27.741°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Argentina, Brazil (type locality), Paraguay.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
Nests in rotten wood.
Castes
Ergatoid queens occur as well as winged queens (Silva & Brandao 2014)
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- rudis. Acanthognathus rudis Brown & Kempf, 1969: 100, figs. 1, 2, 4-10 (w.q.m.) BRAZIL (São Paulo, Rio Grande do Sul, Santa Catarina, Paraná, Rio de Janeiro).
- Type-material: holotype worker, 61+ paratype workers (total not stated), 8 paratype queens, 2 paratype males.
- Type-locality: holotype Brazil: São Paulo, São Paulo, Jardim Botanico, ii.1967 (W.L. Brown); paratypes: workers (number not stated), 1 queen, 2 males with same data as holotype; 61+ workers, 7 queens Brazil: Rio Grande do Sul, Tainhas, iv.1959 (F. Plaumann), Santa Catarina, Chapecó, v, viii.1969 (F. Plaumann), Ibicaré, vii.1959 (F. Plaumann), Ibicaré, ix.1960 (F. Plaumann), Nova Teutonia, vii.1952, x.1953, vi.1957, vii.1957, ii.1959, ii.1960, vi.1960, vii.1961, i.1963, and vi.1963 (F. Plaumann), Santa Catarina, Seara, vii.1958 (F. Plaumann), Paraná, Bocaiuva, v.1963 (F. Plaumann), Palmeira, v. 1963 (F. Plaumann), Volta Grande, iv.1949 (Hertel), São Paulo, Barueri, xii.1958 (K. Lenko), Guararema, xii.1957 (W.W. Kempf), SãoPaulo, Jardim Botanico, Agua Funda, viii.1958 (K. Lenko), Jardim Botanico, viii.1962 and ii.1967 (W.L. Brown), Serra do Cantareia (Kempf & Santos), Boraceia, Salesopolis, ii.1967 (R.H. Crozier), Rio de Janeiro, Parc Nac. do Itatiaia, xii.1955 (T. Borgmeier).
- Type-depositories: MZSP (holotype); MCZC, MZSP (paratypes).
- Wheeler, G.C. & Wheeler, J. 1969: 110 (l.); Silva, T.S.R. & Brandão, 2014: 164 (ergatoid q.).
- Status as species: Kempf, 1972a: 8; Brandão, 1991: 322; Dietz & Brandão, 1993: 683; Bolton, 1995b: 53; Gronenberg, et al. 1998: 227; Bolton, 1999: 1652; Bolton, 2000: 17; Wild, 2007b: 30; Galvis & Fernández, 2009: 249 (in key); Sosa-Calvo, et al. 2010: 39 (in key); Silva, T.S.R. & Brandão, 2014: 164.
- Distribution: Brazil.
Description
Worker
Holotype. TL 4.2, HL 0.93, H\V 0.65 (CI 70), ML 0.68 (MI 73), WL 0.86, petiole L in dorsal view 0.55, postpetiole L 0.20 (W 0.20), gaster L 0.97, greatest diameter of compound eye 0.3, scape L (chord to basal collar) 0.76 mm.
This species is so well known (under the name ocellatus) that Figs. 1, 2, and 4, and the measurements and indices, plus mention of a few outstanding features, should suffice to characterize it. Note the rather V-like outline of the posterior excision of the head. Head slightly more depressed, less convex above, than in A. ocellatus. The outstanding trait is the fine, nearly opaque, densely rugulose punctulate sculpture of head and truncus. Among the rugules are crowded numerous small piligerous fossae, especially on the dorsum of the head, but these are clearly visible only in certain lights. Sides of truncus finely punctulate-rugulose, except for the lowest part of the mesothorax, which is smooth and shining. The hairs are abundant, particularly on head and promesonotum, but also present on nodes, short and inclined, slender but blunt, often feebly flattened or clavate toward their apices, those on the head directed anteriad.
Pilosity otherwise as described for the genus.
Humeral angles obtuse, not strongly projecting. Propodeal teeth diverging, very feebly curved as seen from above.
The mandibles lack preapical armament, and there is not even a distinct welt at the site of the trigger hair, though a formation extending internally to the base of the hair can be seen within the transparent cuticle of the inner mandibula margin.
Petiole with a low, rounded node, the lower anterior slope with a low median carina; node about 0.22 mm long and 0.r 8 mm wide; postpetiole subglobular. Petiole and postpetiole densely and finely reticulo-punctulate and opaque, except for the almost completely smooth, shining nodal summits. Color light reddish ferruginous ; nodes and gaster yellowish ferruginous; but the gaster with the middle third shading into a broad brownish-red transverse band ; appendages yellow to straw.
Paratype variation is slight on the whole. Workers from Boraceia, S. Paulo State, have the upper as well as lower mesopleura largely smooth and shining. Color varies from light to medium ferruginous.
Queen
Measurements of a queen from the type locality are given in Table I. Her distinguishing specific characters correspond in the usual way to those of the worker. Mesonotum with crowded, slightly vermiculate longitudinal rugulae, interspersed with small fossae. Pronotum and propodeum transversely rugulose. Mesopleura with the upper half rugulose or smooth; lower half mostly smooth and shining.
Male
from the type nest series: TL 2.8, HL 0.52, HW without eyes 0.45, with eyes 0.54, L antenna 2.0, ML 0.05, WL 0.90, L forewing 2.55 mm.
Smooth and shining, with small punctures abundant on head, a few rugae around the antennal insertions. Mesonotum finely and indistinctly longitudinally striolate-punctulate, sericeous-opaque. Color brown to piceous, head darkest (specimens may not be fully colored). Legs and mouthparts yellowish-tan.
References
- Bolton, B. 1999. Ant genera of the tribe Dacetonini (Hymenoptera: Formicidae). J. Nat. Hist. 3 33: 1639-1689 (page 1652, see also)
- Bolton, B. 2000. The ant tribe Dacetini. Mem. Am. Entomol. Inst. 65: 1-1028 (page 17, see also)
- Brandão, C. R. F. 1991. Adendos ao catálogo abreviado das formigas da região Neotropical (Hymenoptera: Formicidae). Rev. Bras. Entomol. 35: 319-412 (page 322, catalogue)
- Brown, W. L., Jr. 1988h. Data on Malpighian tubule numbers in ants (Hymenoptera: Formicidae). Pp. 17-27 in: Trager, J. C. (ed.) Advances in myrmecology. Leiden: E. J. Brill, xxvii + 551 pp. (page 23, anatomy)
- Brown, W. L., Jr.; Kempf, W. W. 1969. A revision of the neotropical dacetine ant genus Acanthognathus (Hymenoptera: Formicidae). Psyche (Camb.) 76: 87-109. (page 100, figs. 1, 2, 4-10 worker, queen, male described)
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Dietz, B. H.; Brandão, C. R. F. 1993. Comportamento de caça e dieta de Acanthognathus rudis Brown & Kempf, com comentários sobre a evolução da predação em Dacetini (Hymenoptera, Formicidae, Myrmicinae). Rev. Bras. Entomol. 37: 683-692 (page 683, behavior)
- Feitosa, R.M., Silva, R.R.da, Aguiar, A.P. 2016. Diurnal flight periodicity of a Neotropical ant assemblage (Hymenoptera, Formicidae) in the Atlantic Forest. Revista Brasileira de Entomologia 60, 241–247. (doi:10.1016/j.rbe.2016.05.006).
- Fernández, F.; Palacio, E. E.; Mackay, W. P.; Mackay, E. S. 1996. Introducción al estudio de las hormigas (Hymenoptera: Formicidae) de Colombia. Pp. 349-412 in: Andrade, M. G., Amat García, G., Fernández, F. (eds.) Insectos de Colombia. Estudios escogido (page 381, anatomy, behavior)
- Gronenberg, W.; Brandão, C. R. F.; Dietz, B. H.; Just, S. 1998. Trap-jaws revisited: the mandibular mechanism of the ant Acanthognathus. Physiol. Entomol. 23: 227-240 (page 227, anatomy, behavior)
- Jansen, G., Savolainen, R. 2010. Molecular phylogeny of the ant tribe Myrmicini (Hymenoptera: Formicidae). Zoological Journal of the Linnean Society 160(3), 482–495 (doi:10.1111/j.1096-3642.2009.00604.x).
- Kempf, W. W. 1972b. Catálogo abreviado das formigas da regia~o Neotropical. Stud. Entomol. 15: 3-344 (page 9, catalogue)
- Paul, J. Gronenberg, W. 1999. Optimizing force and velocity: mandible muscle fibre attachments in ants. Journal of Experimental Biology 202, 797-808.
- Silva, T.S.R., Brandao, C.R.F. 2014. Further ergatoid gyne records in the ant tribe Dacetini (Formicidae: Myrmicinae). Neotropical Entomology 43, 161–171 (DOI 10.1007/s13744-013-0192-7).
- Wheeler, G. C.; Wheeler, J. 1969. The larva of Acanthognathus (Hymenoptera: Formicidae). Psyche (Camb.) 76: 110-113 (page 110, larva described)
References based on Global Ant Biodiversity Informatics
- Bihn J. H., M. Verghaagh, M. Brandle, and R. Brandl. 2008. Do secondary forests act as refuges for old growth forest animals? Recovery of ant diversity in the Atlantic forest of Brazil. Biological Conservation 141: 733-743.
- Bolton, B. 2000. The Ant Tribe Dacetini. Memoirs of the American Entomological Institute 65
- Brandao, C.R.F. 1991. Adendos ao catalogo abreviado das formigas da regiao neotropical (Hymenoptera: Formicidae). Rev. Bras. Entomol. 35: 319-412.
- Brown W. L., and W. W. Kempf. 1969. A revision of the Neotropical dacetine ant genus Acanthognathus (Hymenoptera: Formicidae). Psyche 76: 87-109.
- Dietz B. H., and C. R. F. Brandão. 1993. Comportamento de caça e dieta de Acanthognathus rudis Brown & Kempf, com comentários sobre a evolução da predação em Dacetini (Hymenoptera, Formicidae, Myrmicinae). Revista Brasileira de Entomologia 37: 683-692.
- Fernandes T. T., R. R. Silva, D. Rodrigues de Souza-Campana, O. Guilherme Morais da Silva, and M. Santina de Castro Morini. 2019. Winged ants (Hymenoptera: Formicidae) presence in twigs on the leaf litter of Atlantic Forest. Biota Neotropica 19(3): http://dx.doi.org/10.1590/1676-0611-bn-2018-0694
- Fernandes T. T., W. Dattilo, R. R. Silva, P. Luna, C. M. Oliveira, and M. Santina de Castro Morini. 2019. Ant occupation of twigs in the leaf litter of the Atlantic Forest: influence of the environment and external twig structure. Tropical Conservation Science 12: 1-9.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Fleck M. D., E. Bisognin Cantarelli, and F. Granzotto. 2015. Register of new species of ants (Hymenoptera: Formicidae) in Rio Grande do Sul state. Ciencia Florestal, Santa Maria 25(2): 491-499.
- Gronenberg W., C. R. F. Brandão, B. H. Dietz, and S. Just,. 1998. Trap-jaws revisited: the mandibular mechanism of the ant Acanthognathus. Physiological Entomology 23: 227-240.
- Instituto Nacional de Pesquisas de Amazonia (GBIF)
- Kempf W. W. 1978. A preliminary zoogeographical analysis of a regional ant fauna in Latin America. 114. Studia Entomologica 20: 43-62.
- Kempf, W.W. 1972. Catalago abreviado das formigas da regiao Neotropical (Hym. Formicidae) Studia Entomologica 15(1-4).
- Mentone T. O., E. A. Diniz, C. B. Munhae, O. C. Bueno, and M. S. C. Morini. 2011. Composition of ant fauna (Hymenoptera: Formicidae) at litter in areas of semi-deciduous forest and Eucalyptus spp., in Southeastern Brazil. Biota Neotrop. 11(2): http://www.biotaneotropica.org.br/v11n2/en/abstract?inventory+bn00511022011.
- Morini M. S. de C., C. de B. Munhae, R. Leung, D. F. Candiani, and J. C. Voltolini. 2007. Comunidades de formigas (Hymenoptera, Formicidae) em fragmentos de Mata Atlântica situados em áreas urbanizadas. Iheringia, Sér. Zool., Porto Alegre, 97(3): 246-252.
- Oliveira Mentone T. de, E. A. Diniz, C. de Bortoli Munhae, O. Correa Bueno and M. S. de Castro Morini. 2012. Composition of ant fauna (Hymenoptera: Formicidae) at litter in areas of semi-deciduous forest and Eucalyptus spp., in Southeastern Brazil. Biota Neotrop 11(2): 237-246.
- Silva R.R., and C. R. F. Brandao. 2014. Ecosystem-Wide Morphological Structure of Leaf-Litter Ant Communities along a Tropical Latitudinal Gradient. PLoSONE 9(3): e93049. doi:10.1371/journal.pone.0093049
- Silva T. S. R., and C. R. F. Brandao. 2014. Further Ergatoid Gyne Records in the Ant Tribe Dacetini (Formicidae: Myrmicinae). Neotrop. Entomol DOI 10.1007/s13744-013-0192-7
- Souza D. R. de., T. T. Fernandes, J. R. de Oloveira Nascimento, S. S. Suguituru, and M. S. de C. Morini. 2012. Characterization of ant communities (Hymenoptera Formicidae) in twigs in the leaf litter of the Atlantic rainforest and Eucalyptus trees in the southeast region of Brazil. Psyche 2012(532768): 1-12
- Suguituru S. S., D. R. de Souza, C. de Bortoli Munhae, R. Pacheco, and M. S. de Castro Morini. 2011. Diversidade e riqueza de formigas (Hymenoptera: Formicidae) em remanescentes de Mata Atlântica na Bacia Hidrográfica do Alto Tietê, SP. Biota Neotrop. 13(2): 141-152.
- Suguituru S. S., M. Santina de Castro Morini, R. M. Feitosa, and R. Rosa da Silva. 2015. Formigas do Alto Tiete. Canal 6 Editora 458 pages
- Suguituru S. S., R. Rosa Silva, D. R. de Souza, C. de Bortoli Munhae, and M. Santina de Castro Morini. Ant community richness and composition across a gradient from Eucalyptus plantations to secondary Atlantic Forest. Biota Neotrop. 11(1): 369-376.
- Ulyssea M. A., C. R. F. Brandao. 2013. Catalogue of Dacetini and Solenopsidini ant type specimens (Hymenoptera, Formicidae, Myrmicinae) deposited in the Museu de Zoologia da Universidade de Sao Paulo, Brazil. Papies Avulsos de Zoologia 53(14): 187-209.
- Ulyssea M.A., C. E. Cereto, F. B. Rosumek, R. R. Silva, and B. C. Lopes. 2011. Updated list of ant species (Hymenoptera, Formicidae) recorded in Santa Catarina State, southern Brazil, with a discussion of research advances and priorities. Revista Brasileira de Entomologia 55(4): 603-611.
- Wild, A. L. "A catalogue of the ants of Paraguay (Hymenoptera: Formicidae)." Zootaxa 1622 (2007): 1-55.
- da Silva, R.R. and R. Silvestre. 2004. Riqueza da fauna de formigas (Hymenoptera: Formicidae) que habita as camadas superficiais do solo em Seara, Santa Catarina. Papéis Avulsos de Zoologia (São Paulo) 44(1): 1-11