Acanthognathus brevicornis
| Acanthognathus brevicornis | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Myrmicinae |
| Tribe: | Attini |
| Genus: | Acanthognathus |
| Species: | A. brevicornis |
| Binomial name | |
| Acanthognathus brevicornis Smith, M.R., 1944 | |
Very little is known about the biology of these ants. Collections have shown, however, that the colonies are small and that they are found in the soil or in rotting wood. No information is available on feeding habits, but the species of Acanthognathus are probably predaceous like some of their close relatives.
| At a Glance | • Ergatoid queen |
Identification
The length and shape of the antennal scape, and the nature of the sculpturing of the head and thorax readily distinguish the worker of brevicornis from those of the other species.
Keys including this Species
Distribution
Latitudinal Distribution Pattern
Latitudinal Range: 9.154722° to -27.818°.
| North Temperate |
North Subtropical |
Tropical | South Subtropical |
South Temperate |
- Source: AntMaps
Distribution based on Regional Taxon Lists
Neotropical Region: Brazil, Colombia, Ecuador, Guyana, Panama (type locality), Peru.
Distribution based on AntMaps
Distribution based on AntWeb specimens
Check data from AntWeb
Countries Occupied
| Number of countries occupied by this species based on AntWiki Regional Taxon Lists. In general, fewer countries occupied indicates a narrower range, while more countries indicates a more widespread species. |

|
Estimated Abundance
| Relative abundance based on number of AntMaps records per species (this species within the purple bar). Fewer records (to the left) indicates a less abundant/encountered species while more records (to the right) indicates more abundant/encountered species. |

|
Biology
The ants (type material) were collected sometime during the period from June to October 1943, and bear the label, Zetek No. 5105, Nothing is known concerning their biology.
Castes
Ergatoid queens occur as well as winged queens (Silva & Brandao 2014).
Images from AntWeb

| |
| Worker. Specimen code casent0280684. Photographer Estella Ortega, uploaded by California Academy of Sciences. | Owned by NHMUK, London, UK. |
Nomenclature
The following information is derived from Barry Bolton's Online Catalogue of the Ants of the World.
- brevicornis. Acanthognathus brevicornis Smith, M.R. 1944c: 151 (w.q.) PANAMA (Barro Colorado I.).
- Type-material: holotype worker, 3 paratype queens.
- Type-locality: holotype Panama: Canal Zone, Barro Colorado I., vi-x.1943, Zetek No. 5105, USNM 56862 (J. Zetek); paratypes with same data.
- Type-depositories: USNM (holotype); MCZC, USNM (paratypes).
- Silva, T.S.R. & Brandão, 2014: 163 (ergatoid q.).
- Status as species: Kempf, 1964e: 67; Brown & Kempf, 1969: 94 (redescription); Kempf, 1972a: 9; Bolton, 1995b: 53; Bolton, 1999: 1652; Bolton, 2000: 16; Sosa-Calvo, et al. 2006: 820; Galvis & Fernández, 2009: 246; Sosa-Calvo, et al. 2010: 39 (in key); Silva, T.S.R. & Brandão, 2014: 163; Bezděčková, et al. 2015: 114; Fernández & Serna, 2019: 831.
- Distribution: Brazil, Colombia, Guyana, Panama, Peru.
Type Material
Described from a holotype worker and an allotype female. Two paratype females do not differ appreciably from the allotype. All of these are deposited in the United States National Museum under U.S.N.M. No. 56862.
- Holotype, worker, Canal Zone: Barro Colorado Island, Panama, James Zetek, U.S.N.M.No. 56862, National Museum of Natural History.
- Paratype, 3 queens, Canal Zone: Barro Colorado Island, Panama, James Zetek, U.S.N.M.No. 56862, National Museum of Natural History.
Description
Worker
Length (including mandibles) 3 mm.
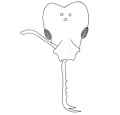
Head subcordate, the posterior border strongly and angularly emarginate. Antenna 11-segmented; scape short (approximately three-fourths the length of the head measured from the anterior border of the clypeus to the posterior corner), not attaining the posterior border at any point, slender, curved and enlarged toward the apex but narrowing again before its junction with the funiculus; first, ninth, and tenth funicular segments long, the second through the eighth short and somewhat, indistinct. Eye placed approximately at the middle of the side of the head, oval, well developed, with about 7 to 8 facets in its greatest diameter. Clypeus longer than broad, subtruncate anteriorly, with the posterior border extending between the frontal carinae to the approximate limits of the latter. Frontal carinae short, each forming a prominent lobe which conceals the insertion of the antenna. Mandibles 0.86 mm. long (slightly shorter than the head), elongate, slender, subparallel, porrect, each with 3 curved, hook-like apical teeth, the median of which is the longest. Inner border of mandible with a slight enlargement near the middle, and a number of very minute denticulae between the enlargement and the apex. Ventral surface of each mandible near the base with a slender, curved spine which is apically bidentate and is directed somewhat mesoposteriorly. Humerus of prothorax with a prominent, tubercle-like spine. Promesonotal suture more or less indistinct. Mesoepinotal impression pronounced. Epinotum higher than long and bearing a pair of well-developed, acutely tipped spines. Petiole strongly pedunculate. Petiole and postpetiole rather nodiform, and without spongiform processes such as occur in Strumigenys.
Head with a shining appearance due to the nature of the sculpturing which consists of rather sparse, subcircular depressions, each bearing a central elevation from which arises a short, curved, bluntly tipped or claviform hair. In the posterior part of the head the punctures are either absent or else separated by a space more than their greatest diameter. All the interspaces are smooth and shining. Thorax so weakly sculptured that it is also shining. Body dark reddish brown with slightly lighter appendages.
Queen
Dealated. Length (including mandibles) 3.85 mm.
Besides the usual caste differences the female differs from the worker in its larger size, more convex and larger eyes (with 12-13 facets in their greatest diameter) and coarser sculpturing on the head. The punctures on the head are not dense enough to give the head a subopaque appearance.
References
- Albuquerque, E., Prado, L., Andrade-Silva, J., Siqueira, E., Sampaio, K., Alves, D., Brandão, C., Andrade, P., Feitosa, R., Koch, E., Delabie, J., Fernandes, I., Baccaro, F., Souza, J., Almeida, R., Silva, R. 2021. Ants of the State of Pará, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001, 1–83 (doi:10.11646/zootaxa.5001.1.1).
- Bolton, B. 1999. Ant genera of the tribe Dacetonini (Hymenoptera: Formicidae). J. Nat. Hist. 3 33: 1639-1689. (page 1652, see also)
- Bolton, B. 2000. The ant tribe Dacetini. Mem. Am. Entomol. Inst. 65: 1-1028 (page 16, see also)
- Brown, W. L., Jr.; Kempf, W. W. 1969. A revision of the neotropical dacetine ant genus Acanthognathus (Hymenoptera: Formicidae). Psyche (Camb.) 76: 87-109. (page 94, redescription of worker and queen)
- Cantone S. 2017. Winged Ants, The Male, Dichotomous key to genera of winged male ants in the World, Behavioral ecology of mating flight (self-published).
- Fernández, F.; Palacio, E. E.; Mackay, W. P.; Mackay, E. S. 1996. Introducción al estudio de las hormigas (Hymenoptera: Formicidae) de Colombia. Pp. 349-412 in: Andrade, M. G., Amat García, G., Fernández, F. (eds.) Insectos de Colombia. Estudios escogido (page 381, see also)
- Franco, W., Ladino, N., Delabie, J.H.C., Dejean, A., Orivel, J., Fichaux, M., Groc, S., Leponce, M., Feitosa, R.M. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674, 509–543 (doi:10.11646/zootaxa.4674.5.2).
- Galvis, J. P. & Fernández, F. 2009. Ants of Colombia X. Acanthognathus with the description of a new species (Hymenoptera: Formicidae) Revista Colombiana de Entomología 35 (2): 245-249.
- Kempf, W. W. 1964e. Miscellaneous studies on Neotropical ants. III. (Hymenoptera: Formicidae). Stud. Entomol. 7: 45-71. (page 67, redescription of worker and queen)
- Kempf, W. W. 1972b. Catálogo abreviado das formigas da regia~o Neotropical. Stud. Entomol. 15: 3-344 (page 9, catalogue)
- Silva, T.S.R., Brandao, C.R.F. 2014. Further ergatoid gyne records in the ant tribe Dacetini (Formicidae: Myrmicinae). Neotropical Entomology 43, 161–171 (DOI 10.1007/s13744-013-0192-7).
- Smith, M. R. 1944c. A key to the genus Acanthognathus Mayr, with the description of a new species (Hymenoptera: Formicidae). Proc. Entomol. Soc. Wash. 46: 150-152. (page 151, worker, queen described)
- Sosa-Calvo, J., T.R. Schultz, and J.S. LaPolla. 2010. A review of the dacetine ants of Guyana (Formicidae: Myrmicinae). Journal of Hymenoptera Research, 19(1): 12-43.
References based on Global Ant Biodiversity Informatics
- Amat-G G., M. G. Andrade-C. and F. Fernández. (eds.) 1999. Insectos de Colombia. Volumen II. Bogotá: Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 433 pp. 131975
- Baroni Urbani C., and M. L. De Andrade. 1994. First description of fossil Dacetini ants with a critical analysis of the current classification of the tribe (Amber Collection Stuttgart: Hymenoptera, Formicidae. VI: Dacetini). Stuttgarter Beiträge zur Naturkunde. Serie B (Geologie und Paläontologie) 198: 1-65.
- Bezdeckova K., P. Bedecka, and I. Machar. 2015. A checklist of the ants (Hymenoptera: Formicidae) of Peru. Zootaxa 4020 (1): 101–133.
- Brown W. L., and W. W. Kempf. 1969. A revision of the Neotropical dacetine ant genus Acanthognathus (Hymenoptera: Formicidae). Psyche 76: 87-109.
- Donoso David. Personal communication. Ants of Barro Colorado Island.
- Feitosa R. dos S. M. and A. S. Ribeiro. 2005. Mirmecofauna (Hymenoptera, Formicidae) de serapilheira de uma área de Floresta Atlântica no Parque Estadual daCantareira São Paulo, Brasil. Biotemas 18: 51-71.
- Fernández, F. and S. Sendoya. 2004. Lista de las hormigas neotropicales. Biota Colombiana Volume 5, Number 1.
- Franco W., N. Ladino, J. H. C. Delabie, A. Dejean, J. Orivel, M. Fichaux, S. Groc, M. Leponce, and R. M. Feitosa. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509-543.
- Groc S., J. H. C. Delabie, F. Fernandez, M. Leponce, J. Orivel, R. Silvestre, Heraldo L. Vasconcelos, and A. Dejean. 2013. Leaf-litter ant communities (Hymenoptera: Formicidae) in a pristine Guianese rainforest: stable functional structure versus high species turnover. Myrmecological News 19: 43-51.
- Instituto Nacional de Pesquisas de Amazonia (GBIF)
- Lapolla, J.S., T. Suman, J. Soso-Calvo and T.R. Schultz. 2006. Leaf litter ant diversity in Guyana. Biodiversity and Conservation 16:491510
- Museo de Entomología de la Universidad del Valle (GBIF)
- Silva R. R., R. S. Machado Feitosa, and F. Eberhardt. 2007. Reduced ant diversity along a habitat regeneration gradient in the southern Brazilian Atlantic Forest. Forest Ecology and Management 240: 61-69.
- Silva R.R., and C. R. F. Brandao. 2014. Ecosystem-Wide Morphological Structure of Leaf-Litter Ant Communities along a Tropical Latitudinal Gradient. PLoSONE 9(3): e93049. doi:10.1371/journal.pone.0093049
- Silva T. S. R., and C. R. F. Brandao. 2014. Further Ergatoid Gyne Records in the Ant Tribe Dacetini (Formicidae: Myrmicinae). Neotrop. Entomol DOI 10.1007/s13744-013-0192-7
- Sosa-Calvo J., T. R. Schultz, and J. S. LaPolla. 2010. A review of the dacetine ants of Guyana (Formicidae: Myrmicinae). Journal of Hymenoptera Research 19: 12-43.